Simplify regulatory searches with PharmaPendium AI

Unfortunately we don't fully support your browser. If you have the option to, please upgrade to a newer version or use Mozilla Firefox, Microsoft Edge, Google Chrome, or Safari 14 or newer. If you are unable to, and need support, please send us your feedback.
We'd appreciate your feedback.Tell us what you think!
You need high-quality data to discover, innovate and develop with confidence. PharmaPendium combines unparalleled regulatory and drug data with full-text search and predictive tools. Reduce animal testing and launch drugs faster with PharmaPendium.

Of utmost importance is PharmaPendium's role in providing information about clinical adverse events, allowing for the identification of underlying toxicity in nonclinical studies.

Guy Bouvier, PhD, ERT
Director, Toxicology & Product Safety at Groupe Pierre Fabre
Predicting pharmacokinetic Drug-Drug Interactions with the maximum of relevance, precision and reactivity is essential. DDIRC enables rapid responses, hence decisions, on the interaction risks.
Yannick Parmentier
Head of the Biopharmaceutical Research Department at Servier
With PharmaPendium, I get information about how it was measured, and a click will show what source document it came from — all very important for building robust, well-founded models.
Senior Modeler
Novartis

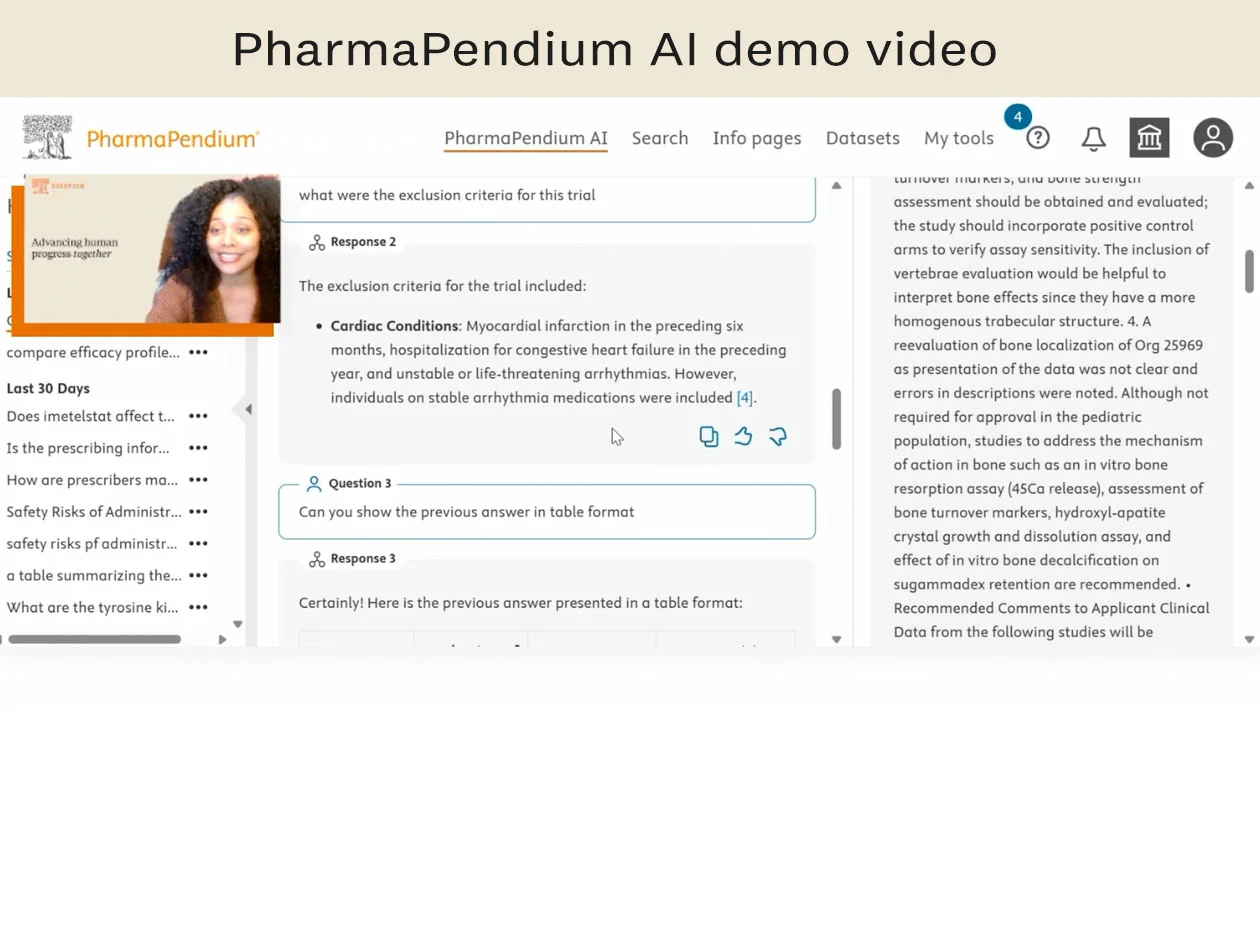
Watch now
|
To advance the best drug candidates, you want to prove better performance. PharmaPendium has user-friendly tools to help you analyze safety, deliverability and efficacy data.
Simplify regulatory searching with PharmaPendium AI, a natural language AI search tool
Predict drug-drug interactions with confidence
Predict the risk of off-target adverse drug reactions (Safety Margin Tool)
Access global data with a translational view across preclinical, clinical and post-market

Minimize regulatory cycling and avoid failing approval with PharmaPendium.
Discover complete regulatory packages from the EMA and FDA, and FDA Advisory Committee Meeting Documents
Search global regulatory guidance from the FDA, EMA and ICH to support compliant drug development decisions
Explore previous regulatory submissions and learn from precedents to predict agencies’ requirements
Benchmark against approved and withdrawn drugs with the same mechanism of action as your drug candidates
Rapidly respond to regulatory questions using comparative data from FDA/EMA drug approval and review documents

Developed in collaboration with Pfizer, Tox Navigator includes toxicity data from regulatory documents and scientific articles.
Supporting the 3Rs and patient safety assessment, the Tox Navigator helps you:
Convert animal doses to human equivalent doses (HEDs)
Leverage existing data to reduce number of animal studies and design more efficient studies
Investigate adverse drug reactions across different species
Integrate in silico models and other non-animal testing methods
Optimize research efforts by focusing on candidates with favorable safety profiles

Advance your research with Elsevier’s PharmaPendium and a portfolio of solutions for pharmaceutical R&D.
Innovate with confidence, supported by:
Trusted quality information from regulatory data to peer-reviewed scientific literature
Innovative technology that powers data transformation, and analytical and predictive tools
Domain and data science expertise to solve complex problems with data solutions for R&D
Information integrity is essential to your progress. Discover trusted data and tools that deliver critical insights.
Let's shape progress together.
... PharmaPendium excels in retrieving specific toxicity observations across approval documents categorized by drug and species.
Guy Bouvier, PhD, ERT
Director, Toxicology & Product Safety at Groupe Pierre Fabre
Continuous development and improvement of PharmaPendium has been achieved in collaboration with the FDA for 17 years, as well as with leading pharma partners like Novartis, Sanofi, Merck, Servier and Boehringer Ingelheim.
PharmaPendium is used by:
Toxicologists and safety pharmacologists
DMPK specialists
Clinical researchers
Regulatory affairs experts
Global patient safety experts
Data scientists
PharmaPendium provides you with unique content, all in one place. PharmaPendium data sources include:
Full FDA approval packages
Full EMA approval documents
FDA Advisory Committee Documents
FDA Adverse Event Reporting System (FAERS)
FDA classic collection (covering 1938-1991)
DESI (Drug Efficacy Study Implementation) documents
Meyler's 16th Edition
Mosby's Drug Consult™️
Scientific articles
We extract, organize, connect and continually update a wide variety data in PharmaPendium. Get the latest content statistics for PharmaPendium.
PharmaPendium includes safety, PK, MET, efficacy, activity, FAERS and drug data, as well as approval packages and documents from the FDA and EMA.
PharmaPendium and Embase combined empower you to find additional indications for drug repurposing of unapproved or approved drugs. With the breadth of data in Embase and PharmaPendium, you can find relevant clinical studies and data to predict the requirements of clinical trial design and to mitigate risk. In addition to data on approved drugs in PharmaPendium, Embase provides information on unapproved drugs. Embase covers more than 8,500 scientific journals and millions of abstracts from 11,500 conferences worldwide.
