快速获取关键的监管见解
Watch now
|
使用 PharmaPendium AI 帮助用户做出明智的药物管道和项目决策。利用自然语言处理和机器学习,提取关键的监管见解。
借助这一新工具,监管事务、药物开发和临床研究专业人员可以:
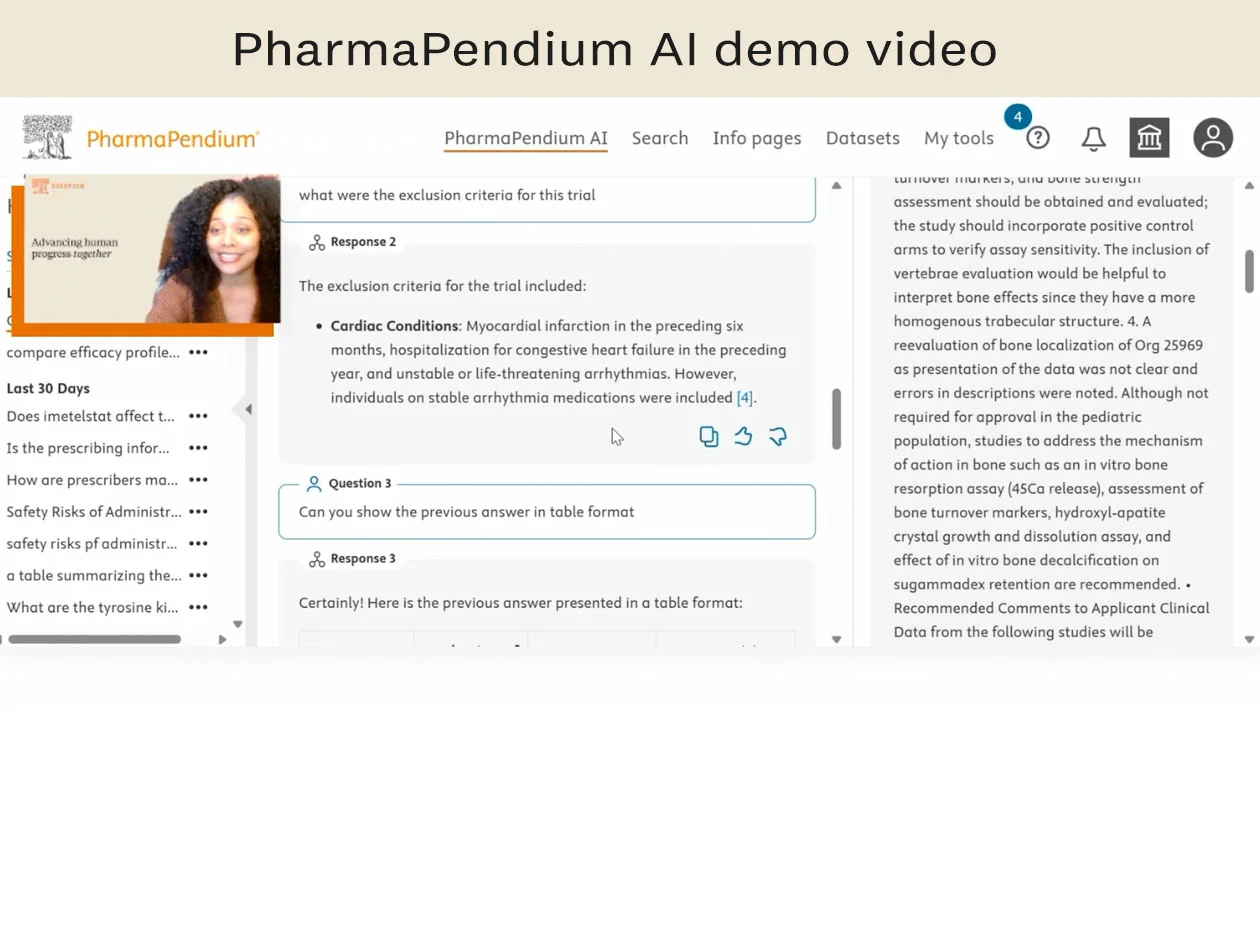
用自然语言提出问题。
从每周更新的 500 万页经过验证的 FDA 和 EMA 文件中获取回复。
引用研究信息的链接。
提出后续问题。
导出会话以轻松维护记录。
使用 PharmaPendium AI 走得更远、更快。
非常抱歉,我们不完全支持您的浏览器。如果您可以选择,请升级到较新版本或使用 Mozilla Firefox、Microsoft Edge、Google Chrome 或 Safari 14 或更高版本。如果您无法进行此操作且需要支持,请将您的反馈发送给我们。
PharmaPendium AI 是一种自然语言 AI 搜索工具,可根据 FDA 和 EMA 的全面监管数据进行搜索。快速、深入的响应揭示了监管先例,帮助监管事务和研发专业人员降低遗漏重要信息的风险。使用 PharmaPendium AI 优化监管策略。


Watch now
|
使用 PharmaPendium AI 帮助用户做出明智的药物管道和项目决策。利用自然语言处理和机器学习,提取关键的监管见解。
借助这一新工具,监管事务、药物开发和临床研究专业人员可以:
用自然语言提出问题。
从每周更新的 500 万页经过验证的 FDA 和 EMA 文件中获取回复。
引用研究信息的链接。
提出后续问题。
导出会话以轻松维护记录。
使用 PharmaPendium AI 走得更远、更快。

用自己的话提问,以更快地找到监管先例。使用后续问题进行深入挖掘。
通过多种语言的自然语言搜索更好地支持全球团队,包括:
英语
中文
法语
German
Japanese
Korean
西班牙语
您的搜索是私密的。PharmaPendium AI 是根据爱思唯尔的负责任 AI 原则和隐私原则开发的,不会将信息存储在 LLM 中,也不会将其用于训练目的。

PharmaPendium AI 根据监管内容中排名前 15 的相关文件提供摘要。根据以下内容继续做出回应:
自 1938 年以来的 FDA 批准包
自 1995 年以来的 EMA 批准文件
自 1983 年以来的 FDA 咨询委员会文件
梅勒药物的副作用第 16 版

PharmaPendium AI 帮助监管事务专业人士以及临床前和临床研究人员预测风险并减少监管周期。
加强监管规划和提交,并做出回应:
基于权威监管术语
可通过原始监管文件的链接进行验证
保存在您的历史记录中以供将来参考
以灵活的格式交付,包括:
桌子
自由文本
要点
CSV 表格
PharmaPendium AI 用户对它如何节省时间并帮助他们做出明智的决策印象深刻。
“快速获取监管先例,这可以帮助我设计更好的(非临床)策略。” — 中型制药公司非临床毒理学和 DMPK 主管
“节省时间,加快流程,减少人为错误的可能性。” — 大型制药公司医疗联盟运营负责人
“减少时间工作,增加知识,并有助于决策和监管策略。” — 中型制药公司监管事务官
Discover how PharmaPendium AI advances drug development by empowering pharmaceutical and biotech teams with rapid, data-driven insights. Watch a webinar recording from September 23, 2025.
Enhance regulatory success with PharmaPendium AI
