Simplify regulatory searches with PharmaPendium AI

Infelizmente, não oferecemos suporte total ao seu navegador. Se for possível, atualize para uma versão mais recente ou use o Mozilla Firefox, o Microsoft Edge, o Google Chrome ou o Safari 14 ou mais recente. Se não conseguir e precisar de suporte, envie seu feedback.
Gostaríamos de receber seu feedback sobre essa nova experiência.Diga-nos sua opinião
O PharmaPendium combina dados regulatórios e de medicamentos incomparáveis com ferramentas preditivas e de pesquisa de texto completo. Reduza os testes em animais e lance medicamentos mais rapidamente com o PharmaPendium.

PharmaPendium é essencial para fornecer informações sobre eventos adversos clínicos e identificar a toxicidade subjacente em estudos não clínicos.

Guy Bouvier, PhD, ERT
Diretor de Toxicologia e Segurança de Produtos em Groupe Pierre Fabre
Prever interações farmacocinéticas com máxima relevância, precisão e agilidade é essencial. O DDIRC permite respostas rápidas e decisões sobre riscos de interação.
Yannick Parmentier
Chefe de Pesquisa Biofarmacêutica em Servier
Com o PharmaPendium, obtenho informações sobre como foi medido e acesso ao documento-fonte com um clique — essencial para modelos robustos e bem fundamentados.
Senior Modeler
Novartis

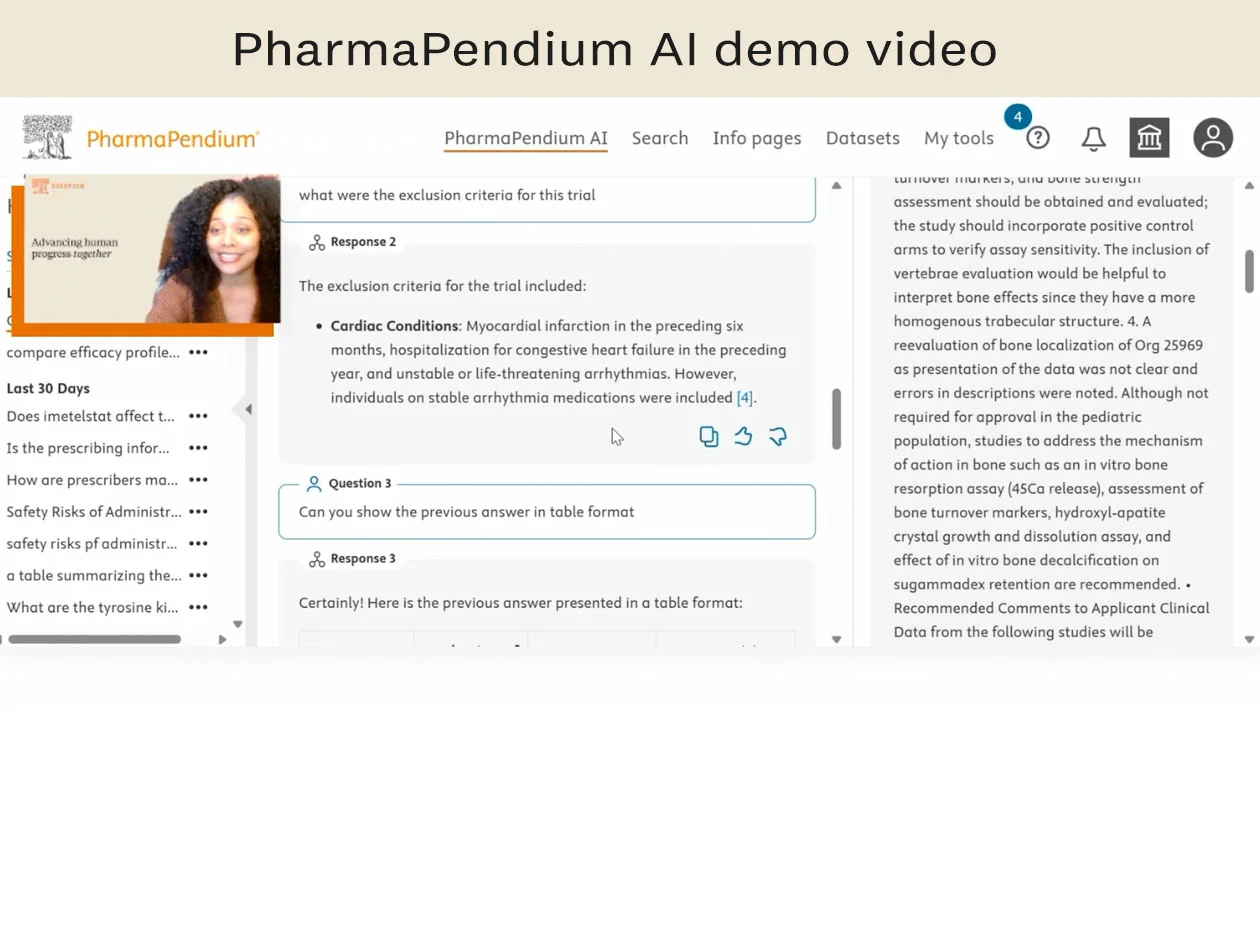
Watch now
|
Para promover os melhores candidatos a medicamentos, você deseja provar um melhor desempenho. O PharmaPendium possui ferramentas fáceis de usar para ajudá-lo a analisar dados de segurança, capacidade de entrega e eficácia.
Preveja interações medicamentosas com confiança
Prever o risco de reações adversas a medicamentos fora do alvo (margem de segurança)
Acesse os dados, incluindo:
Segurança pré-clínica e clínica
Modelos animais
Eventos adversos (MedDRA)
Parâmetros farmacocinéticos
Metabolismo e transporte
Ensaio clínico e desfechos
Relatórios pós-comercialização (FAERS)

Minimize o ciclo regulatório e evite falhas na aprovação com o PharmaPendium.
Descubra pacotes regulatórios completos da EMA e FDA e documentos da reunião do Comitê Consultivo da FDA
Pesquise orientações regulatórias globais da FDA, EMA e ICH para apoiar decisões conformes no desenvolvimento de medicamentos
Explore as submissões regulatórias anteriores e aprenda com os precedentes para prever os requisitos das agências
Compare com medicamentos aprovados e retirados com o mesmo mecanismo de ação que seus candidatos a medicamentos
Responda rapidamente a perguntas regulatórias usando dados comparativos de documentos de aprovação e revisão de medicamentos da FDA/EMA

Developed in collaboration with Pfizer, Tox Navigator includes toxicity data from regulatory documents and scientific articles.
Supporting the 3Rs and patient safety assessment, the Tox Navigator helps you:
Convert animal doses to human equivalent doses (HEDs)
Leverage existing data to reduce number of animal studies and design more efficient studies
Investigate adverse drug reactions across different species
Integrate in silico models and other non-animal testing methods
Optimize research efforts by focusing on candidates with favorable safety profiles

Advance your research with Elsevier’s PharmaPendium and a portfolio of solutions for pharmaceutical R&D.
Innovate with confidence, supported by:
Trusted quality information from regulatory data to peer-reviewed scientific literature
Innovative technology that powers data transformation, and analytical and predictive tools
Domain and data science expertise to solve complex problems with data solutions for R&D
Information integrity is essential to your progress. Discover trusted data and tools that deliver critical insights.
Let's shape progress together.
... O PharmaPendium se destaca na recuperação de observações específicas de toxicidade em documentos de aprovação categorizados por medicamento e espécie.
Guy Bouvier, PhD, ERT
Diretor de Toxicologia e Segurança de Produtos em Groupe Pierre Fabre
Continuous development and improvement of PharmaPendium has been achieved in collaboration with the FDA for 17 years, as well as with leading pharma partners like Novartis, Sanofi, Merck, Servier and Boehringer Ingelheim.
PharmaPendium is used by:
Toxicologists and safety pharmacologists
DMPK specialists
Clinical researchers
Regulatory affairs experts
Global patient safety experts
Data scientists
PharmaPendium provides you with unique content, all in one place. PharmaPendium data sources include:
Full FDA approval packages
Full EMA approval documents
FDA Advisory Committee Documents
FDA Adverse Event Reporting System (FAERS)
FDA classic collection (covering 1938-1991)
DESI (Drug Efficacy Study Implementation) documents
Meyler's 16th Edition
Mosby's Drug Consult™️
Scientific articles
We extract, organize, connect and continually update a wide variety data in PharmaPendium. Get the latest content statistics for PharmaPendium.
PharmaPendium includes safety, PK, MET, efficacy, activity, FAERS and drug data, as well as approval packages and documents from the FDA and EMA.
PharmaPendium and Embase combined empower you to find additional indications for drug repurposing of unapproved or approved drugs. With the breadth of data in Embase and PharmaPendium, you can find relevant clinical studies and data to predict the requirements of clinical trial design and to mitigate risk. In addition to data on approved drugs in PharmaPendium, Embase provides information on unapproved drugs. Embase covers more than 8,500 scientific journals and millions of abstracts from 11,500 conferences worldwide.
