How to close the carbon loop with CO2 conversion
14 de junio de 2023
Por Chris Cogswell, PhD

Learn about key processes and technologies that power carbon capture, utilization and storage (CCUS) projects
Sustainability, a circular economy, the closed carbon loop — these terms weren’t on the radar of most organizations in the energy and natural resource space a few decades ago. Now they’re the goal.
Carbon capture, utilization and storage (CCUS) technologies are the driving force behind a changing economy. Developed to reduce carbon emissions and reach net zero targets, these technologies are also shifting industry perspectives from CO2 as a waste product to CO2 as a valuable resource. The carbon utilization component of CCUS is an ever-evolving field of research focused on building viable, cost-effective methods of converting CO2 into high-value products.
An acute understanding of the different technologies, materials and products involved in the CO2 conversion process helps organizations define their CCUS project goals, driving innovation and economic success.
Thermocatalysis
Thermocatalysis uses heat and pressure to convert CO2 into alcohols like methanol and ethanol by reacting it with hydrogen, usually at temperatures of 700°C to 1000°C. These alcohols can then be used to manufacture biofuels for transportation companies and heating and electricity applications.

Sustainable Utilization of CO2 toward a Circular Economy: Prospects, Challenges, and Opportunities (Access on Knovel)
CO2 hydrogenation, also called methanation, is a promising method of thermocatalysis for the production of methanol. It’s traditionally a two-step process that begins by reducing CO2 to CO, then combining it with hydrogen to create hydrocarbon fuels like methane, ethane and propane. To streamline the process and make it more efficient, researchers are searching for ways to reduce it to a single reaction. One prototype from a group of Stanford University engineers used ruthenium and iron oxide nanoparticles as the catalyst with successful results.
Carbon Recycling International demonstrates the power of hydrogenation on a large scale by manufacturing two types of methanol via thermocatalysis:
Renewable methanol
Recycled carbon methanol
Renewable methanol, also called e-methanol, combines CO2 captured from flue streams from industrial processes with hydrogen produced with water electrolysis using a renewable electricity source. The gas is then fed into the reactor, where a catalytic conversion transforms it into crude methanol, sending it to the distillation unit for purification. Recycled carbon methanol, also known as low carbon methanol, follows the same process, with the only difference being that the hydrogen comes from waste streams.
One downside of thermocatalysis is the need for hydrogen as an input, typically acquired through a methane-reforming process requiring high temperatures — which produces added CO2. A potential solution is to produce hydrogen through a water-splitting process or to source it from waste residue — like Carbon Recycling International does — to create a greener product.
Electrochemical conversion
Electrochemical conversion, or electrocatalysis, utilizes electricity to power catalytic converters. This adjustment allows electrochemical conversion to become a completely carbon-neutral option for CO2 conversion when using renewable energy sources like solar or wind power.
Better conditions are another advantage of electrochemical conversion because cells don’t require thermal-level temperatures and pressure. Electrochemical cells are also designed to be small, simple and less expensive than bulky thermal reactors.
Through the electrochemical conversion of CO2, organizations can manufacture various gas products like carbon monoxide, methane and hydrogen and liquid products like methanol and formic acid. These can then become the inputs of usable products like fuels and household goods.

Source: A Comprehensive Review on Different Approaches for CO2 Utilization and Conversion Pathways, Chemical Engineering Science (June 2021)
The problem with electrochemical conversion is its low energy efficiency and insufficient chemistry control, making commercialization difficult. To address this issue, researchers aim to improve the process through two types of modifications. The first field of research focuses on improving the catalysts, like combining copper with other metals for better selectivity. The second aims to redesign the electrochemical cell, for example, using a solid electrolyte to transport ions instead of liquid.
Despite the current commercial limitations of electrochemical conversion, California-based Twelve is on a mission to make this technology a reality. The company has partnered with NASA, Alaska Airlines and Virgin Airlines to produce CO2-made jet fuel. This year, Twelve is launching Opus, an “industrial-scale … platform designed to handle the demands of the world's biggest brands.”
Photocatalysis
Photocatalysis is a novel method that replaces electricity or heat in the catalysis process with solar energy to create a completely carbon-negative conversion similar to the photosynthesis found in the natural carbon cycle.
The four-step process begins with catalyst activation via a UV or visible light, causing electrons to shift from the valence band to the conduction band. The empty space on the valence band gathers electron and hole pairs, which reduce the CO2 to create products like carbon monoxide and methane.

Source: Photocatalytic Conversion of Carbon Dioxide: From Products to Design the Catalysts, Journal of CO2 Utilization (Dec 2019)
Currently, photocatalysis is a heavily researched area of study with little large-scale application, but Syzygy Plasmonics is one of the few organizations successfully utilizing this technology to convert CO2. The company currently makes two products — syngas and hydrogen — using photocatalysts. Syngas and hydrogen are then further transformed into low-carbon fuels, methanol and fertilizers.
The significant advantage of photocatalysis is that it’s carbon-neutral or carbon-negative if combined with a CO2 capture project. Using renewable resources like solar energy to power the conversion process can also reduce the operational costs of supplying electricity or heat to other catalytic methods. Still, photocatalysis remains mainly in the research and development phase because photocatalysts tend to be unstable, provide weak absorption and often have poor selectivity.
Biocatalysis
Biocatalysis substitutes chemical catalysts with enzymes or microorganisms within the catalytic conversion process. It’s widely used in the pharmaceutical industry to produce chemicals for the manufacture of medicine, but researchers are testing methods to expand the process to encompass carbon-based products like fuel and plastics.
Using microalgae biomass for photosynthesis of CO2 to produce biofuels and other high-value products is a particular field of interest for researchers due to microalgae’s natural CO2 conversion response.
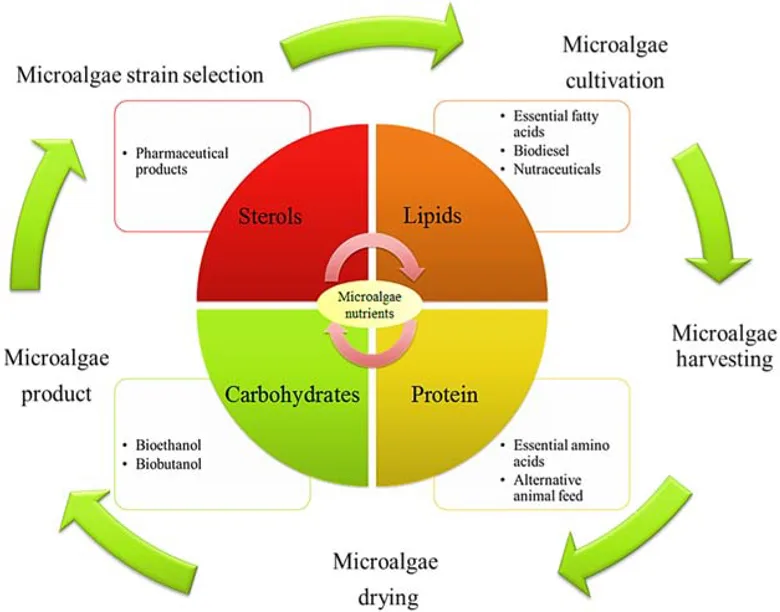
Source: Upcycling of Carbon from Waste via Bioconversion into Biofuel and Feed (Access on Knovel)
Illinois-based LanzaTech uses a different biocatalyst — rabbit-gut bacteria — to convert CO2 into products like ethanol and ethylene. The latter is a component of polyethylene, a polymer with many use cases, from fabric and plastics to household products. LanzaTech already works with several global brands to produce low-carbon products using their solutions, including jet fuels for Virgin Airlines and All Nippon Airways, plastic packaging for L’Oreal, and fragrances for Coty. They also partner with multiple clothing manufacturers, including Lululemon and Zara, to produce polyester fabric. You can see a diagram of their process here.
One significant advantage of biocatalysis is its ability to create reactions under low temperatures and pressure while maintaining high selectivity. Key challenges toward wide-scale adoption include the costs associated with cell materials and low production rates, which make it difficult to scale up the process. Electricity consumption is another concern, but renewable energy sources can counterbalance this issue and ensure carbon neutrality.
Carbon mineralization
Carbon mineralization is often used for CO2 sequestration within geological formations, but researchers are now looking at this option as a utilization opportunity. It mimics the natural process of carbonation with an accelerated timeframe. Captured CO2 is reacted with minerals, like calcium oxide, to create carbonates used in the manufacturing of high-value products.
Carbon8 and Blue Planet Systems are two organizations using carbon mineralization to produce low-carbon building materials, like aggregate and concrete, on a large scale. Both companies use captured CO2 directly from flue streams and other waste residues to create carbonates onsite within modular facilities. This method removes the need for purifying, transporting and storing CO2, which streamlines the conversion process and lowers operational costs.
Carbon mineralization can also be used to produce household products like baking soda. Texas-based CarbonFree launched their industrial-scale facility, SkyMine, in 2016. The carbon mineralization plant “is capable of capturing up to 50,000 metric tons of CO2 per year” and converting it into sodium bicarbonate, or baking soda.
Not only does this process produce a high-value product, but it locks CO2 into storage permanently. Carbon mineralization of CO2 also contributes to lower resource waste when using recycled concrete as an added material to save water and sand. The downsides of this method center around its limited potential for CO2 storage. For example, Carbon8 can capture and process around 1,500 to 4,000 tons of CO2 per year — a minuscule fraction of the 10 Gt of CO2 scientists say we need to target annually.
Close the carbon loop with data insights
The idea of a closed carbon loop, or a sustainable circular economy, is inching closer to becoming a reality for many industries. An ever-growing collection of cutting-edge research produced by experts worldwide that’s focused on the conversion of CO2 is driving this shift. Focus your data discovery process with Knovel, a knowledge platform designed specifically for engineers.
Learn more about CO2 conversion through Knovel’s platform, which features technical content from 160 validated sources and over 75 million data points, to see how it can power your organization’s sustainability transformation and CCUS project goals.
Contribuidor

Chris Cogswell, PhD
Customer and Engineering Global Consultant
Elsevier
Leer más acerca de Chris Cogswell, PhD