Guide for authors
- Introduction
- Before you begin
- Ethics in publishing
- Declaration of interest
- Declaration of generative AI in scientific writing
- Submission declaration and verification
- Preprint posting on SSRN
- Use of inclusive language
- Reporting sex- and gender-based analyses
- Author contributions
- Changes to authorship
- Copyright
- Open access
- Submission
- Categories
- Preparation
- Manuscript preparation
- Language
- Article Layout
- Title and Abstract
- Keywords
- Acknowledgments
- Nomenclature and abbreviations
- GenBank
- Equations and formulae
- Footnotes
- Figure Legends
- Tables
- References
- Graphical Abstract
- Research data
- Research Elements
- After acceptance
Introduction
Biochemical Pharmacology is an international peer reviewed journal devoted to publishing original research and invited reviews and commentaries on the interaction of chemical compounds with biological systems. Manuscripts describing experiments conducted with chemical mixtures, plant or animal extracts will not be considered for publication unless the chemical structures and precise concentrations of all substances are reported.
While particular emphasis is placed on reporting findings that relate to pharmacodynamics, pharmacokinetics, and metabolism of both small molecules and biologics at the biochemical and molecular levels, submissions in the areas of behavioral and physiological pharmacology and toxicology are considered if they describe studies directed at defining mechanisms of action. All areas related to the field of pharmacology are represented in the journal including, but not limited to, chemotherapy, neuropharmacology, inflammation/immunopharmacology, antimicrobials, behavioral, respiratory, gastrointestinal, cardiovascular and endocrine pharmacology and toxicology.
Reports describing de novo results of clinical studies and those that predominately or exclusively concern database mining and analysis and computational methodologies, e.g. CAMD, are outside the scope of the journal.
Types of papers(1) Full-length Research Papers. Biochemical Pharmacology publishes original research on issues of relevance to the field of pharmacology.
(2) Review Articles. Biochemical Pharmacology publishes reviews on topics of interest to pharmacologists. While these articles should be as concise as possible, they must include information and interpretations representing different points of view. Reviews can vary in length from 4,000 to 25,000 words, not including references. Inclusion of tables and diagrams as figures is encouraged. Besides being balanced and accurate in the presentation of data, reviews must be authoritative, state-of-the-art accounts of subjects of topical interest to investigators in the field. Articles that simply summarize published reports without proposing new interpretations, experimental approaches or therapeutic implications will not be considered for publication. As with regular research reports, review articles undergo rigorous peer review to determine their suitability for publication in Biochemical Pharmacology.
Manuscript preparation and submission
Provided below is detailed information on the scientific criteria and manuscript formatting required for an article to be considered for publication in Biochemical Pharmacology. The online submission process includes the Scientific Submission Checklist (Table 1) on the 'Additional Information Screen' at https://www.editorialmanager.com/bp/default.aspx. Failure to accurately complete the Scientific Submission Checklist questions automatically disqualifies the work for consideration. See Mullane et al., Guidelines for Manuscript Submission in the Peer-Reviewed Pharmacological Literature(Biochem. Pharmacol 97:225-235, 2015; https://www.sciencedirect.com/science/article/pii/S0006295215003585) for a detailed discussion of the issues addressed in the Scientific Submission Checklist.
Table 1. Scientific Submission Checklist
Please answer the following questions with "Yes", "No", or "Not applicable".
Formatting – Only video or audio files may be uploaded as supplementary data. The submission will automatically be rejected if the first question is marked "no" (i.e. supplementary tables or figures are not permitted).
1. As Biochemical Pharmacology does NOT publish supplemental data with the exception of audio or video files, are all necessary data included in the body of the manuscript?
2. Are all tables and figures numbered and appropriately titled with descriptive legends that permit stand-alone interpretation? Are all data shown in the tables and figures also described in the Results section, discussed in the Discussion section and stated in the Conclusions?
Introduction Section
3. Is there a clear statement with background describing the hypothesis being tested by this study? Are the primary endpoints clearly stated?
Materials and Methods Section
4. Were human tissues or fluids used in this study? Were the experiments reviewed and approved by the Institutional review Board (IRB)?
5. Were animals used in the study? Has the species, strain, sex, weight and source of the animals been provided? If used, is the method of anesthesia described? Were the experiments reviewed and approved by the Instructional Animal Care and Use Committee (IACUC).
6. Is justification provided in the text for examining only a single rather than two or more cell lines?
7. Are the source(s) and passage number of cell lines indicated and authenticated by you or the vendor?
8. Is (are) the chemical structure(s) of any new compound(s) presented as a figure or referenced in the peer-reviewed literature?
9. Are the sources of all materials clearly indicated? If used, has the selectivity of antibodies and/or interference RNA been validated and their source clearly indicated?
10. Is the rationale for the selection of concentrations, doses, route and frequency of compound administration provided?
11. Are quantified results (e.g., IC50 and/or EC50 values) of concentration- and dose-response experiments included in the manuscript?
12. Are all group sizes approximately the same and clearly indicated in the text and/or in the tables and figures?
13. Were the criteria used for excluding any data from analysis determined prospectively and clearly stated?
14. Was the investigator responsible for data analysis blinded to which samples/animals represent control and treatment groups?
15. Are the reported data displayed as means +/- standard deviation (SD)? Is the number of replicates of three or more independent experimental observations clearly indicated? Were post-hoc tests used to assess the statistical significance among means? Is the threshold for statistical significance (P value) clearly indicated?
Results Section
16. If western blots are shown, are the appropriate loading controls, replication data, and quantification and statistical analysis shown?
17. If PCR and RT-PCR are included, were MIQE guidelines followed? Was a reference standard (positive or negative controls) included in the study to validate the experiment?
Discussion Section
18. Are the primary conclusions and any secondary endpoints and their implications clearly stated?
19. Are the limitations of the current study or alternative interpretations of the findings clearly stated?
Conflict of Interest/Financial Support
20. Is a conflict of interest statement included in the manuscript?
21. Are all organizations providing funding for this work listed in Acknowledgements? In the online submission system, please list any additional explanation(s) you feel may be necessary on the above questions.
Please list any additional explanation(s) you feel may be necessary on the above questions:
Before you begin
Ethics in publishingPlease see our information on Ethics in publishing. Declaration of interest
All authors must disclose any financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work. Examples of potential competing interests include employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding. Authors must disclose any interests in two places: 1. A summary declaration of interest statement in the title page file (if double anonymized) or the manuscript file (if single anonymized). If there are no interests to declare then please state this: 'Declarations of interest: none'. 2. Detailed disclosures as part of a separate Declaration of Interest form, which forms part of the journal's official records. It is important for potential interests to be declared in both places and that the information matches. More information. Declaration of generative AI in scientific writing
The below guidance only refers to the writing process, and not to the use of AI tools to analyse and draw insights from data as part of the research process.
Where authors use generative artificial intelligence (AI) and AI-assisted technologies in the writing process, authors should only use these technologies to improve readability and language. Applying the technology should be done with human oversight and control, and authors should carefully review and edit the result, as AI can generate authoritative-sounding output that can be incorrect, incomplete or biased. AI and AI-assisted technologies should not be listed as an author or co-author, or be cited as an author. Authorship implies responsibilities and tasks that can only be attributed to and performed by humans, as outlined in Elsevier’s AI policy for authors.
Authors should disclose in their manuscript the use of AI and AI-assisted technologies in the writing process by following the instructions below. A statement will appear in the published work. Please note that authors are ultimately responsible and accountable for the contents of the work.
Disclosure instructions
Authors must disclose the use of generative AI and AI-assisted technologies in the writing process by adding a statement at the end of their manuscript in the core manuscript file, before the References list. The statement should be placed in a new section entitled ‘Declaration of Generative AI and AI-assisted technologies in the writing process’.
Statement: During the preparation of this work the author(s) used [NAME TOOL / SERVICE] in order to [REASON]. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
This declaration does not apply to the use of basic tools for checking grammar, spelling, references etc. If there is nothing to disclose, there is no need to add a statement.
Submission declaration and verificationSubmission of an article implies that the work described has not been published previously (except in the form of an abstract, a published lecture or academic thesis, see 'Multiple, redundant or concurrent publication' for more information), that it is not under consideration for publication elsewhere, that its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder. To verify compliance, your article may be checked by Crossref Similarity Check and other originality or duplicate checking software. Preprints
Please note that preprints can be shared anywhere at any time, in line with Elsevier's sharing policy. Sharing your preprints e.g. on a preprint server will not count as prior publication (see 'Multiple, redundant or concurrent publication' for more information). Preprint posting on SSRN
In support of Open Science, this journal offers its authors a free preprint posting service. Preprints provide early registration and dissemination of your research, which facilitates early citations and collaboration.
During submission to Editorial Manager, you can choose to release your manuscript publicly as a preprint on the preprint server SSRN once it enters peer-review with the journal. Your choice will have no effect on the editorial process or outcome with the journal. Please note that the corresponding author is expected to seek approval from all co-authors before agreeing to release the manuscript publicly on SSRN.
You will be notified via email when your preprint is posted online and a Digital Object Identifier (DOI) is assigned. Your preprint will remain globally available free to read whether the journal accepts or rejects your manuscript.
For more information about posting to SSRN, please consult the SSRN Terms of Use and FAQs.
Use of inclusive languageInclusive language acknowledges diversity, conveys respect to all people, is sensitive to differences, and promotes equal opportunities. Content should make no assumptions about the beliefs or commitments of any reader; contain nothing which might imply that one individual is superior to another on the grounds of age, gender, race, ethnicity, culture, sexual orientation, disability or health condition; and use inclusive language throughout. Authors should ensure that writing is free from bias, stereotypes, slang, reference to dominant culture and/or cultural assumptions. We advise to seek gender neutrality by using plural nouns ("clinicians, patients/clients") as default/wherever possible to avoid using "he, she," or "he/she." We recommend avoiding the use of descriptors that refer to personal attributes such as age, gender, race, ethnicity, culture, sexual orientation, disability or health condition unless they are relevant and valid. When coding terminology is used, we recommend to avoid offensive or exclusionary terms such as "master", "slave", "blacklist" and "whitelist". We suggest using alternatives that are more appropriate and (self-) explanatory such as "primary", "secondary", "blocklist" and "allowlist". These guidelines are meant as a point of reference to help identify appropriate language but are by no means exhaustive or definitive. Reporting sex- and gender-based analyses
Reporting guidance
For research involving or pertaining to humans, animals or eukaryotic cells, investigators should integrate sex and gender-based analyses (SGBA) into their research design according to funder/sponsor requirements and best practices within a field. Authors should address the sex and/or gender dimensions of their research in their article. In cases where they cannot, they should discuss this as a limitation to their research's generalizability. Importantly, authors should explicitly state what definitions of sex and/or gender they are applying to enhance the precision, rigor and reproducibility of their research and to avoid ambiguity or conflation of terms and the constructs to which they refer (see Definitions section below). Authors can refer to the Sex and Gender Equity in Research (SAGER) guidelines and the SAGER guidelines checklist. These offer systematic approaches to the use and editorial review of sex and gender information in study design, data analysis, outcome reporting and research interpretation - however, please note there is no single, universally agreed-upon set of guidelines for defining sex and gender.
Definitions
Sex generally refers to a set of biological attributes that are associated with physical and physiological features (e.g., chromosomal genotype, hormonal levels, internal and external anatomy). A binary sex categorization (male/female) is usually designated at birth (""sex assigned at birth""), most often based solely on the visible external anatomy of a newborn. Gender generally refers to socially constructed roles, behaviors, and identities of women, men and gender-diverse people that occur in a historical and cultural context and may vary across societies and over time. Gender influences how people view themselves and each other, how they behave and interact and how power is distributed in society. Sex and gender are often incorrectly portrayed as binary (female/male or woman/man) and unchanging whereas these constructs actually exist along a spectrum and include additional sex categorizations and gender identities such as people who are intersex/have differences of sex development (DSD) or identify as non-binary. Moreover, the terms ""sex"" and ""gender"" can be ambiguous—thus it is important for authors to define the manner in which they are used. In addition to this definition guidance and the SAGER guidelines, the resources on this page offer further insight around sex and gender in research studies.
For transparency, we require corresponding authors to provide co-author contributions to the manuscript using the relevant CRediT roles. The CRediT taxonomy includes 14 different roles describing each contributor’s specific contribution to the scholarly output. The roles are: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Roles/Writing - original draft; and Writing - review & editing. Note that not all roles may apply to every manuscript, and authors may have contributed through multiple roles. More details and an example. Changes to authorship
Authors are expected to consider carefully the list and order of authors before submitting their manuscript and provide the definitive list of authors at the time of the original submission. Any addition, deletion or rearrangement of author names in the authorship list should be made only before the manuscript has been accepted and only if approved by the journal Editor. To request such a change, the Editor must receive the following from the corresponding author: (a) the reason for the change in author list and (b) written confirmation (e-mail, letter) from all authors that they agree with the addition, removal or rearrangement. In the case of addition or removal of authors, this includes confirmation from the author being added or removed.
Only in exceptional circumstances will the Editor consider the addition, deletion or rearrangement of authors after the manuscript has been accepted. While the Editor considers the request, publication of the manuscript will be suspended. If the manuscript has already been published in an online issue, any requests approved by the Editor will result in a corrigendum. Article transfer service
This journal uses the Elsevier Article Transfer Service to find the best home for your manuscript. This means that if an editor feels your manuscript is more suitable for an alternative journal, you might be asked to consider transferring the manuscript to such a journal. The recommendation might be provided by a Journal Editor, a dedicated Scientific Managing Editor, a tool assisted recommendation, or a combination. If you agree, your manuscript will be transferred, though you will have the opportunity to make changes to the manuscript before the submission is complete. Please note that your manuscript will be independently reviewed by the new journal. More information. Copyright
Upon acceptance of an article, authors will be asked to complete a 'Journal Publishing Agreement' (see more information on this). An e-mail will be sent to the corresponding author confirming receipt of the manuscript together with a 'Journal Publishing Agreement' form or a link to the online version of this agreement.
Subscribers may reproduce tables of contents or prepare lists of articles including abstracts for internal circulation within their institutions. Permission of the Publisher is required for resale or distribution outside the institution and for all other derivative works, including compilations and translations. If excerpts from other copyrighted works are included, the author(s) must obtain written permission from the copyright owners and credit the source(s) in the article. Elsevier has preprinted forms for use by authors in these cases.
For gold open access articles: Upon acceptance of an article, authors will be asked to complete a 'License Agreement' (more information). Permitted third party reuse of gold open access articles is determined by the author's choice of user license.
Author rights
As an author you (or your employer or institution) have certain rights to reuse your work. More information.
Find out how you can share your research published in Elsevier journals. Open access
Please visit our Open Access page for more information about open access publishing in this journal. Submission
All articles must be submitted online at https://www.editorialmanager.com/bp/default.aspx. Initial manuscripts may be submitted as pdfs but revised manuscripts must be uploaded as separate editable files (e.g. text and tables in Word or LaTeX and figures in common artwork formats - seehttps://www.elsevier.com/about/policies-and-standards/author/artwork-and-media-instructions). Institutional Email Address
As of January 1st, 2016 manuscripts will not be considered for publication in Biochemical Pharmacology if the email address for the corresponding author does not reflect an affiliation with a research-based institution. The institutional email address MUST be included in the registered profile of the corresponding author in Editorial Manager, the online submission system. Alternatively, the submission must be accompanied by a separate statement in English on institutional letterhead, which is signed by an official responsible for research activities for the institute from which the manuscript originates, verifying the corresponding author is affiliated with the research institution. The statement must also include official's institutional email address and full contact information. Suggesting reviewers
Please submit the names and institutional e-mail addresses of several potential reviewers.
You should not suggest reviewers who are colleagues, or who have co-authored or collaborated with you during the last three years. Editors do not invite reviewers who have potential competing interests with the authors. Further, in order to provide a broad and balanced assessment of the work, and ensure scientific rigor, please suggest diverse candidate reviewers who are located in different countries/regions from the author group. Also consider other diversity attributes e.g. gender, race and ethnicity, career stage, etc. Finally, you should not include existing members of the journal's editorial team, of whom the journal are already aware.
Note: the editor decides whether or not to invite your suggested reviewers.
CategoriesAuthors must indicate on the title page which of the following categories best describes their work:
•Antibiotics and Chemotherapeutics
•Cardiovascular Pharmacology
•Gastrointestinal Pharmacology
•Inflammation and Immunopharmacology
•Metabolic Disorders and Endocrinology
•Neuropharmacology
•Pharmacokinetics and Drug Metabolism
•Pulmonary, Renal and Hepatic Pharmacology
•Toxicology
Preparation
Manuscript preparation LanguageNeither the Editorial Board nor the reviewers will provide detailed advice for improving the grammar and clarity of a manuscript, regardless of the scientific merit of the work. Authors are responsible for ensuring the article is written in clear English. Either American or British usage is accepted, but not a combination of the two. The use of spell-check and grammar-check offered in the word processing software is highly recommended. Manuscripts lacking linguistic clarity or that are not prepared according to the style guidelines outlined below will not be considered for publication. Authors can have their manuscript language-edited. Please check the section "Author resources" for information on language editing. Article Layout
Reports must be written in English with the pages numbered sequentially. The text must be double-spaced in single-column format with 1" or 25 mm margins. Size 12 (point) Times New Roman or Arial font is preferred. The article must be divided into clearly defined and numbered sections. The required sections are 1. Introduction, 2. Materials and Methods, 3. Results, 4. Discussion, and References. See Mullane et al., Guidelines for Manuscript Submission in the Peer-Reviewed Pharmacological Literature (Biochem. Pharmacol.97:225-235, 2015; https://www.sciencedirect.com/science/article/pii/S0006295215003585) for a detailed discussion of the topics that must be covered in each section. Subsections should be numbered 1.1 (then 1.1.1, 1.1.2, ?), 1.2, etc. The abstract is not included in section numbering. This numbering should be used for internal cross-referencing in the text. Subsections may be assigned a brief heading that appears alone on a separate line. Title and Abstract
The article title should be concise but informative. All abbreviations must be spelled out fully when first mentioned in the abstract or body of the report. Abstracts are limited to 250 words. Keywords
Immediately following the abstract the authors must provide up to 6 keywords for indexing purposes. American spelling must be used, avoiding general and plural terms and multiple concepts. Only established abbreviations may be proposed as keywords. Acknowledgments
Acknowledgments must be listed in a separate section at the end of the article before the references. The Acknowledgments should include the names of individuals, organizations and funding agencies that provided assistance in underwriting and reporting the work. Nomenclature and abbreviations
Receptor and ion channel nomenclature must conform to guidelines of the Committee on Receptor Nomenclature and Drug Classification of the International Union of Basic and Clinical Pharmacology (IUPHAR) (http://www.guidetopharmacology.org/nomenclature.jsp). Use only abbreviations that are generally accepted by the scientific community. Click HERE to view the full list of abbreviations that can be employed without definition. Following the abstract and keywords, provide an alphabetical list of all other abbreviations with their definitions that are used throughout the paper, including the tables, figure legends, and figures. Drugs or other compounds should only be identified by their chemical or generic names. The source, including company name and location, for all chemicals, reagents, cell lines, tissue, and experimental animals must be provided in Materials and Methods.
Cell lines
For consideration by the journal a study must include examination of two or more authenticated cell lines to verify results unless the use of a single cell line is adequately justified in the text. Cell line authentication requires specification of 1) source, 2) method of authentication, and 3) passage number.
GenBankGenBank accession numbers should be typed in bold, underlined text. Letters in the accession number should always be capitalized. Example: “…a B-cell tumor from a chronic lymphatic leukemia (GenBank accession no. BE675048), and a T-cell lymphoma (GenBank accession no. AA361117)…” In the final electronic version of the article, the accession number text will be linked to the appropriate source in the NCBI databases. Equations and formulae
Equations and formulae should be typed and numbered consecutively with Arabic numerals in parentheses on the right hand side of the page (if referred to explicitly in the text).
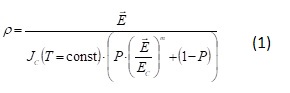
They should be separated before and after the surrounding text by one line.
FootnotesFootnotes should be used sparingly and numbered consecutively throughout the text. Indicate the position of footnotes in the text and list them separately at the end of the article. Do not include footnotes in the Reference list. Figure Legends
Illustrations must have a caption that is listed separately from the figure in the submitted version of the work. The caption should be self-explanatory without the need to reference the accompanying text. All symbols appearing on the illustration must be clearly defined in the figure legend. Tables
All tables must be numbered consecutively in Arabic numerals and cited in the text in their order of appearance. Table titles should be brief and descriptive. Tables should appear individually on separate pages in the submitted version of the work, together with a legend that includes sufficient information about the experimental protocol and results so the reader does not have to refer back to the text to understand the experimental protocol and findings. Tables should not have vertical lines, and the number of horizontal lines should be minimized. References Citations in text
Ensure that every reference cited in the text is present in the reference list, and vice versa. Unpublished results and personal communications should not appear in the reference list, but may be indicated in the text. Data References
While Biochemical Pharmacology does not publish supplemental tables or figures, it is acknowledged that some relevant datasets are too large to print in a volume of the journal. Elsevier collaborates with a number of repositories to link articles on ScienceDirect with extensive datasets, giving readers access to underlying data too large to print to offer a better understanding of the research described. Cite underlying or relevant datasets in your text and include a data reference in your Reference List. Data references should include the following elements: author name(s), dataset title, data repository, version (where available), year, and global persistent identifier. Add [dataset] immediately before the reference so we can properly identify it as a data reference. The [dataset] identifier will not appear in your published article.
[dataset] [5] M. Oguro, S. Imahiro, S. Saito, T. Nakashizuka, Mortality data for Japanese oak wilt disease and surrounding forest compositions, Mendeley Data, v1, 2015. http://dx.doi.org/10.17632/xwj98nb39r.1
For supported data repositories a repository banner will automatically appear next to your published article on ScienceDirect.
In addition, you can link to relevant data or entities too large to print through identifiers within the text of your manuscript, using the following format: Database: xxxx (e.g., TAIR: AT1G01020; CCDC: 734053; PDB: 1XFN).
Preprint referencesWhere a preprint has subsequently become available as a peer-reviewed publication, the formal publication should be used as the reference. If there are preprints that are central to your work or that cover crucial developments in the topic, but are not yet formally published, these may be referenced. Preprints should be clearly marked as such, for example by including the word preprint, or the name of the preprint server, as part of the reference. The preprint DOI should also be provided. Reference formatting
There are no strict requirements on reference formatting for the initial submission. References can be in any style or format as long as they are consistent within the manuscript. Regardless of the format, author(s) name(s), journal title/book title, chapter title/article title, year of publication, volume number/book chapter and the pagination must be shown. Use of DOI is encouraged. Reference management software
Most Elsevier journals have a standard template available in key reference management packages. This covers packages using the Citation Style Language, such as Mendeley (http://www.mendeley.com/features/reference-manager/: Elsevier [Numeric, with titles]) and also others like EndNote (http://endnote.com/downloads/style/biochemical-pharmacology) http://refman.com/downloads/styles/. Graphical Abstract
Authors asked to submit a revised version of the work for consideration must also supply a graphical abstract at that time. The graphical abstract, which will be displayed on the online Table of Contents, should provide a concise summary of the work in pictorial form designed to capture the attention of a wide audience and for compilation of databases. Graphical Abstract text should not exceed 30 words. The content of the graphical abstract must be kept within an area of 5 cm tall by 17 cm wide (landscape shape). Authors are encouraged to limit graphical abstracts to 189 pixels tall by 642 pixels wide to ensure the image and text will be legible when displayed online. Existing landscape-oriented figures are welcomed as graphical abstracts once converted to these proportions. Authors must supply the graphic separately as an electronic file. See https://www.sciencedirect.com/science/journal/00062952/94/2 for examples of graphical abstracts. Research data
This journal requires and enables you to share data that supports your research publication where appropriate, and enables you to interlink the data with your published articles. Research data refers to the results of observations or experimentation that validate research findings, which may also include software, code, models, algorithms, protocols, methods and other useful materials related to the project.
Below are a number of ways in which you can associate data with your article or make a statement about the availability of your data when submitting your manuscript. When sharing data in one of these ways, you are expected to cite the data in your manuscript and reference list. Please refer to the "References" section for more information about data citation. For more information on depositing, sharing and using research data and other relevant research materials, visit the research data page.
Research ElementsThis journal enables you to publish research objects related to your original research – such as data, methods, protocols, software and hardware – as an additional paper in a Research Elements journal.
Research Elements is a suite of peer-reviewed, open access journals which make your research objects findable, accessible and reusable. Articles place research objects into context by providing detailed descriptions of objects and their application, and linking to the associated original research articles. Research Elements articles can be prepared by you, or by one of your collaborators.
During submission, you will be alerted to the opportunity to prepare and submit a manuscript to one of the Research Elements journals.
More information can be found on the Research Elements page.
After acceptance
Online proof correctionTo ensure a fast publication process of the article, we kindly ask authors to provide us with their proof corrections within two days. Corresponding authors will receive an e-mail with a link to our online proofing system, allowing annotation and correction of proofs online. The environment is similar to MS Word: in addition to editing text, you can also comment on figures/tables and answer questions from the Copy Editor. Web-based proofing provides a faster and less error-prone process by allowing you to directly type your corrections, eliminating the potential introduction of errors.
If preferred, you can still choose to annotate and upload your edits on the PDF version. All instructions for proofing will be given in the e-mail we send to authors, including alternative methods to the online version and PDF.
We will do everything possible to get your article published quickly and accurately. Please use this proof only for checking the typesetting, editing, completeness and correctness of the text, tables and figures. Significant changes to the article as accepted for publication will only be considered at this stage with permission from the Editor. It is important to ensure that all corrections are sent back to us in one communication. Please check carefully before replying, as inclusion of any subsequent corrections cannot be guaranteed. Proofreading is solely your responsibility. Offprints
The corresponding author will, at no cost, receive a customized Share Link providing 50 days free access to the final published version of the article on ScienceDirect. The Share Link can be used for sharing the article via any communication channel, including email and social media. For an extra charge, paper offprints can be ordered via the offprint order form which is sent once the article is accepted for publication. Corresponding authors who have published their article gold open access do not receive a Share Link as their final published version of the article is available open access on ScienceDirect and can be shared through the article DOI link. Useful Links
Listed below are links to sites that provide additional details on topics relating to the preparation and submission of manuscripts for publication in Biochemical Pharmacology as well as post-publication resources.
English Editing Services: https://webshop.elsevier.com/language-editing/
Electronic Artwork: https://www.elsevier.com/about/policies-and-standards/author/artwork-and-media-instructions
Reference Formatting: http://endnote.com/downloads/style/biochemical-pharmacology
Acceptable Abbreviations: https://legacyfileshare.elsevier.com/promis_misc/bcp_abbreviations.pdf
Database Linking: https://www.elsevier.com/databaselinking
Commercial Reprints: https://www.elsevier.com/advertising-reprints-supplements
Reprint Permissions: https://www.elsevier.com/permissions
