Guide for authors
- Introduction
- Before you begin
- Submission declaration
- Ethics in publishing
- Declaration of competing interest
- Image integrity and standards
- Studies involving humans
- Studies involving animals
- Declaration of generative AI in scientific writing
- Use of inclusive language
- Reporting sex- and gender-based analyses
- Author contributions
- Open access
- Preparation
- Your Paper Your Way
- Peer review
- Revised submissions
- Essential title page information
- Abstracts
- Main Text
- Keywords
- Abbreviations
- Tables
- References
- Formatting requirements
- Language usage and editing service
- Supplementary data
- Graphical abstract
- Highlights
- Footnotes
- Artwork
- Data visualization
- Research data
- Submission Checklist
- Co-submission
- Changes to authorship
- Copyright
- Role of the funding source
- After acceptance
- Author inquiries
Introduction
Atherosclerosis is a fully electronic journal, all manuscripts are to be submitted via the internet. To submit your paper online, click on the link https://www.editorialmanager.com/ath/default.aspx. Types of paper
Types of papers that can be submitted for consideration by the Editorial Board include:
Original Research Papers are divided into three categories:
- Basic Research Papers reporting results of original research or investigation using in vitro cell culture or animal models.
- Clinical and Population Research Papers reporting results of investigation in human subjects including observational, interventional and genetic studies. Meta-analyses and genetic association studies will also be published under this category. For publication of clinical trials, genetic association studies and meta-analyses, please consult the dedicated Special Guidelines below.
- Translational Research Papers reporting results of research from both bench-to-bedside and bedside-to-bench.
The following word limits apply: abstract 250 words, main text 4000 words (including legends to figures and tables), 5 figures and/or tables in total (authors are encouraged to include additional figures and tables as Supplementary Material) and a maximum of 50 references. Flexibility on word count may be offered after discussion with the Editor.
Methodology papers. They describe novel methods or innovative modifications and applications of existing methods for epidemiological, clinical or experimental research on atherosclerosis or vascular biology. The following word limits apply: abstract 150 words, main text 3000 words (including legends to figures and tables), 3 figures/tables in total and a maximum of 25 references.
Rapid Communications. Atherosclerosis welcomes submissions of manuscripts previously rejected by high-quality journals because of priority reasons as Rapid Communications. Please submit your manuscript together with a cover letter, the reviewers' comments and your rebuttal indicating any revisions made to the manuscript via the journal submission system (https://www.editorialmanager.com/ath) by choosing Rapid Communication as the article type. Your manuscript will be assessed by the Editor in Chief, Co-Editors, and Associate Editors, who will decide within 10 days whether the paper is accepted or not, with or without any revision.
Review Articles. Atherosclerosis publishes review articles on topics of great interest or controversy in basic, translational, clinical or population research. Authors who have not been priorly invited to submit a Review by the Editors of Atherosclerosis are advised to write a letter of interest to the Editorial Office, accompanied by an abstract. Based on this, the Editors will encourage or discourage submission. Please note that we only consider Reviews from authors who contributed significant original research to the reviewed research field; a list of previously published works should be provided in the cover letter. In all cases, Review Articles undergo peer review. The following word limits apply: abstract 250 words, main text 5000 words, 6 figures and/or tables in total, and a maximum of 100 references. Authors are encouraged to include a "mechanism/overview" figure and one or more bullet point boxes highlighting the main key-points.
Clinical and Scientific Debates on Atherosclerosis. In this review, two antipodal experts are invited to debate their opposing views on a relevant topic, where every argument is discussed by the author in favour and the author against. Debates articles will consist of an abstract (250 words), a pro section (2500 words) and a con section (2500 words). A total of 6 figures/tables is accepted. References should not exceed a maximum of 100.
Conference reports. Conference reports are accepted for publication in our Journal and should be structured as follows: 1) authors and contact details (postal address of all authors and email address of corresponding author); 2) name of the conference and name of the organizing national atherosclerosis society); 3) conference dates and venue, and website address if available; 4) name(s) of conference President(s); 5) topics covered by the conference as bullet points; 6) conference highlights (prosa or bullet points); 7) conflict of interest statement with respect to the congress (e.g. sponsorship). 1 one-column width figure is allowed. The word count of the entire report (items 1 through 6) shall not exceed 450 words.
Editorials and Correspondence. Editorials will be commissioned by the Editors, who will approach a suitably qualified author to write a commentary on a recently accepted Original Research Paper of particular interest. Editorials should not exceed 1500 words and 20 references. 1 figure or table is allowed.
If you have specific issues that you wish to raise concerning work published in Atherosclerosis, please submit your opinions as a Correspondence. Correspondence articles should not exceed 1000 words (including references), 10 references and 2 figures and/or tables. The inclusion of novel data will increase the chance of acceptance. The Author(s) of the commented manuscript will have the opportunity to respond to the comments in the same issue of the Journal. Please submit Correspondence to the Editor-in-Chief Arnold von Eckardstein.
In each issue, the following manuscripts will be made available free of charges online:
- Up to 2 articles selected by the Editor-in-Chief
- Invited reviews
- Editorials
Editor-in-Chief
Professor Arnold von Eckardstein
Institute of Clinical Chemistry
University Hospital and University of Zurich
Rämistrasse 100, Zurich
CH-8091
Switzerland
Fax: +41442554590
E-mail: [email protected]
Before you begin
Submission declarationSubmission of an article to Atherosclerosis implies that the work described has not been published previously, except in the form of an abstract or as part of a published lecture or academic thesis.
Submission of an article therefore means:
- The article is not under consideration for publication elsewhere.
- Publication of the article is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out.
- If the article is accepted, it will not be published elsewhere by the authors, including electronically in the same form, in English or in any other language, without the written consent of the copyright-holder.
Atherosclerosis will not tolerate plagiarism in any form in submitted manuscripts. Passages of text, data or figures quoted or closely paraphrased from other authors (or from any part of the author's own published work) must be identified as quotations or paraphrases and the sources of such material must be acknowledged. The use of unacknowledged material will be construed as plagiarism. If any manuscript is found to contain plagiarised material the review process will be halted immediately, and the University or Institute of the corresponding Author will be informed.
Atherosclerosis will not tolerate manipulation or enhancement of data. Authors will be asked to provide further evidence for the validity of data, and the University or Institute of the corresponding Author will be informed if such evidence is not forthcoming.
Ethics in publishingPlease see our information on Ethics in publishing.
Atherosclerosis will not tolerate plagiarism in any form in submitted manuscripts. Text copied from copyrighted works from third parties or from the author's own published work, in any section of the manuscript, is unacceptable. Each submission to Atherosclerosis undergoes a check for plagiarism. Manuscripts with excessive overlap with previously published articles are rejected without peer review. To avoid the overlap between your manuscript and previously published works, please cite the relevant articles as references. Declaration of competing interest
All authors must disclose any financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work. Examples of potential conflicts of interest include employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding. Authors should complete the declaration of competing interest statement using this template and upload to the submission system at the Attach/Upload Files step. Note: Please do not convert the .docx template to another file type. Author signatures are not required. If there are no interests to declare, please choose the first option in the template. More information. Image integrity and standards
While it is accepted that authors sometimes need to manipulate images for clarity, manipulation for purposes of deception or fraud will be seen as scientific ethical abuse and will be dealt with accordingly. For graphical images, Atherosclerosis is applying the following policy:
no specific feature within an image may be enhanced, obscured, moved, removed, or introduced.
Adjustments of brightness, contrast, or color balance are acceptable if and as long as they are done in the whole figure (not in specific parts of it) and do not obscure or eliminate any information present in the original.
Nonlinear adjustments (e.g., changes to gamma settings) must be disclosed in the figure legend. Grouping of images from parts of a gel/different gels must be made explicit.
Studies involving humans
Manuscripts reporting data from research conducted on humans must include a statement of assurance in the Materials and methods section of the manuscript reading that: (1) written informed consent was obtained from each patient included in the study, (2) the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and (3) the study protocol has been priorly approved by the Institution's ethics committee on research on humans.
Identifying information shall not be included in any part of the paper, unless it is essential for scientific purposes and written informed consent for publication in print and electronic version has been obtained. If such consent has not been obtained, personal details of patients included in any part of the paper and in any Supplementary Material must be removed from the submission.
If identifying characteristics are altered to protect anonymity, authors should provide assurance that alterations do not distort scientific meaning and editors should note so. For publication of clinical trials, please consult the dedicated Special Guidelines below.
Studies involving animalsAll experiments on live vertebrates or higher invertebrates must be performed in accordance with relevant institutional and national guidelines and regulations. A statement identifying the committee approving the experiments and confirming that all experiments conform to the relevant regulatory standards must be included in the Materials and methods section of the submission. We suggest that researchers carrying out experiments with animals refer to the ARRIVE guidelines and recommendations developed by the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) to improve experimental design and reporting of animal research. Declaration of generative AI in scientific writing
The below guidance only refers to the writing process, and not to the use of AI tools to analyse and draw insights from data as part of the research process.
Where authors use generative artificial intelligence (AI) and AI-assisted technologies in the writing process, authors should only use these technologies to improve readability and language. Applying the technology should be done with human oversight and control, and authors should carefully review and edit the result, as AI can generate authoritative-sounding output that can be incorrect, incomplete or biased. AI and AI-assisted technologies should not be listed as an author or co-author, or be cited as an author. Authorship implies responsibilities and tasks that can only be attributed to and performed by humans, as outlined in Elsevier’s AI policy for authors.
Authors should disclose in their manuscript the use of AI and AI-assisted technologies in the writing process by following the instructions below. A statement will appear in the published work. Please note that authors are ultimately responsible and accountable for the contents of the work.
Disclosure instructions
Authors must disclose the use of generative AI and AI-assisted technologies in the writing process by adding a statement at the end of their manuscript in the core manuscript file, before the References list. The statement should be placed in a new section entitled ‘Declaration of Generative AI and AI-assisted technologies in the writing process’.
Statement: During the preparation of this work the author(s) used [NAME TOOL / SERVICE] in order to [REASON]. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
This declaration does not apply to the use of basic tools for checking grammar, spelling, references etc. If there is nothing to disclose, there is no need to add a statement.
Use of inclusive languageInclusive language acknowledges diversity, conveys respect to all people, is sensitive to differences, and promotes equal opportunities. Content should make no assumptions about the beliefs or commitments of any reader; contain nothing which might imply that one individual is superior to another on the grounds of age, gender, race, ethnicity, culture, sexual orientation, disability or health condition; and use inclusive language throughout. Authors should ensure that writing is free from bias, stereotypes, slang, reference to dominant culture and/or cultural assumptions. We advise to seek gender neutrality by using plural nouns ("clinicians, patients/clients") as default/wherever possible to avoid using "he, she," or "he/she." We recommend avoiding the use of descriptors that refer to personal attributes such as age, gender, race, ethnicity, culture, sexual orientation, disability or health condition unless they are relevant and valid. When coding terminology is used, we recommend to avoid offensive or exclusionary terms such as "master", "slave", "blacklist" and "whitelist". We suggest using alternatives that are more appropriate and (self-) explanatory such as "primary", "secondary", "blocklist" and "allowlist". These guidelines are meant as a point of reference to help identify appropriate language but are by no means exhaustive or definitive. Reporting sex- and gender-based analyses
Reporting guidance
For research involving or pertaining to humans, animals or eukaryotic cells, investigators should integrate sex and gender-based analyses (SGBA) into their research design according to funder/sponsor requirements and best practices within a field. Authors should address the sex and/or gender dimensions of their research in their article. In cases where they cannot, they should discuss this as a limitation to their research's generalizability. Importantly, authors should explicitly state what definitions of sex and/or gender they are applying to enhance the precision, rigor and reproducibility of their research and to avoid ambiguity or conflation of terms and the constructs to which they refer (see Definitions section below). Authors can refer to the Sex and Gender Equity in Research (SAGER) guidelines and the SAGER guidelines checklist. These offer systematic approaches to the use and editorial review of sex and gender information in study design, data analysis, outcome reporting and research interpretation - however, please note there is no single, universally agreed-upon set of guidelines for defining sex and gender.
Definitions
Sex generally refers to a set of biological attributes that are associated with physical and physiological features (e.g., chromosomal genotype, hormonal levels, internal and external anatomy). A binary sex categorization (male/female) is usually designated at birth (""sex assigned at birth""), most often based solely on the visible external anatomy of a newborn. Gender generally refers to socially constructed roles, behaviors, and identities of women, men and gender-diverse people that occur in a historical and cultural context and may vary across societies and over time. Gender influences how people view themselves and each other, how they behave and interact and how power is distributed in society. Sex and gender are often incorrectly portrayed as binary (female/male or woman/man) and unchanging whereas these constructs actually exist along a spectrum and include additional sex categorizations and gender identities such as people who are intersex/have differences of sex development (DSD) or identify as non-binary. Moreover, the terms ""sex"" and ""gender"" can be ambiguous—thus it is important for authors to define the manner in which they are used. In addition to this definition guidance and the SAGER guidelines, the resources on this page offer further insight around sex and gender in research studies.
For transparency, we require corresponding authors to provide co-author contributions to the manuscript using the relevant CRediT roles. The CRediT taxonomy includes 14 different roles describing each contributor’s specific contribution to the scholarly output. The roles are: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Roles/Writing - original draft; and Writing - review & editing. Note that not all roles may apply to every manuscript, and authors may have contributed through multiple roles. More details and an example. Elsevier supports responsible sharing
Find out how you can share your research published in Elsevier journals. Open access
Please visit our Open Access page for more information about open access publishing in this journal.
Preparation
Your Paper Your WayWe now differentiate between the requirements for new and revised submissions. You may choose to submit your manuscript as a single Word or PDF file to be used in the refereeing process. Only when your paper is at the revision stage, will you be requested to put your paper in to a 'correct format' for acceptance and provide the items required for the publication of your article.
To find out more, please visit the Preparation section below. Peer review
This journal operates a single blind review process. All contributions will be initially assessed by the editor for suitability for the journal. Papers deemed suitable are then typically sent to a minimum of two independent expert reviewers to assess the scientific quality of the paper. The Editor is responsible for the final decision regarding acceptance or rejection of articles. The Editor's decision is final. REVISED SUBMISSIONS
Please ensure the Atherosclerosis style is followed for all revised papers and the checklist uploaded with the revised manuscript Atherosclerosis Checklist. Essential title page information
• Title. Concise and informative. Titles are often used in information-retrieval systems. Avoid abbreviations and formulae where possible.
• Author names and affiliations. Please clearly indicate the given name(s) and family name(s) of each author and check that all names are accurately spelled. You can add your name between parentheses in your own script behind the English transliteration. Present the authors' affiliation addresses (where the actual work was done) below the names. Indicate all affiliations with a lower-case superscript letter immediately after the author's name and in front of the appropriate address. Provide the full postal address of each affiliation, including the country name and, if available, the e-mail address of each author.
• Corresponding author. Clearly indicate who will handle correspondence at all stages of refereeing and publication, also post-publication. This responsibility includes answering any future queries about Methodology and Materials. Ensure that the e-mail address is given and that contact details are kept up to date by the corresponding author.
• Present/permanent address. If an author has moved since the work described in the article was done, or was visiting at the time, a 'Present address' (or 'Permanent address') may be indicated as a footnote to that author's name. The address at which the author actually did the work must be retained as the main, affiliation address. Superscript Arabic numerals are used for such footnotes. Abstracts
A structured Abstract must be provided to include the following four sections: Background and aims, Methods, Results; Conclusions (a maximum of 250 words for regular original research papers). Main Text
The manuscript main text must be structured to include the following sections in this order (please do not deviate from the headers provided):
- Introduction
- Materials and methods (or Patients and methods)
- Results
- Discussion
- Conflict of interest (mandatory)
- Financial support (if applicable)
- Author contributions (mandatory)
- Acknowledgements (if applicable)
- References
A keyword summary must be provided; normally 3-7 items should be included. Abbreviations
Abbreviations should be defined when first used in the text. Use of abbreviations should be kept at a minimum. Tables
Tables must be submitted as Word files. Tables with titles and legends must be on separate pages with double spacing; they may be included in the same file as the manuscript text or in separate file(s). Authors must list on the title page or in the covering e-mail, the number of figures and/or tables to be found in the paper.
Footnotes to tables
Footnotes to tables must be listed with superscript lowercase letters, beginning with "a."
Footnotes must not be listed with numbers or symbols.
Figure and table legends
Each figure and table legend should have a brief overarching title (with figure number) that describes the entire figure without citing specific panels, followed by a description of each panel, and all symbols used.
If a figure or table contains multiple panels, the letter describing each panel should be capitalized and surrounded by parenthesis: i.e. (A)(B)(C)(D).
There are no strict requirements on reference formatting at submission. References can be in any style or format as long as the style is consistent. Where applicable, author(s) name(s), journal title/book title, chapter title/article title, year of publication, volume number/book chapter and the pagination must be present. For authors' name, the general rule is up to 5 names before et al. Use of DOI is highly encouraged. The reference style used by the journal will be applied to the accepted article by Elsevier at the proof stage. Note that missing data will be highlighted at proof stage for the author to correct. Formatting requirements
Units: Units must be expressed following the international system of units (SI). If other units are mentioned, conversion factors into SI units must be provided.
DNA and protein sequences: Gene names should be italicized; protein products of the loci are not italicized.
For murine models, the gene and protein names are lowercase except for the first letter(e.g. gene: Abcb4; protein: Abcb4).
For humans, the whole gene name is capitalized(e.g. gene: ABCB4; protein ABCB4).
Only gene names approved by the HUGO Gene Nomenclature Committee should be used: www.genenames.org.
Mouse strains and cell lines: Knock-out or transgenic mouse strains and cell lines are italicized and the symbol superscripted (e.g. ob/ob , p53+/+, p53-/-).
p values: p values must be consistently formatted according to the below style throughout the manuscript (including figures and tables):
p
p=X
All p are italicized and lower case.
Language usage and editing service: American or British English should be used, but not a mixture of them.
Words must be written consistently in the same way throughout the manuscript (e.g. non-significant or nonsignificant; down-regulation or downregulation). Authors who feel their English language manuscript may require editing to eliminate possible grammatical or spelling errors and to conform to correct scientific English may wish to use the English Language Editing service available from Elsevier's WebShop: https://webshop.elsevier.com/language-editing/ or visit our customer support site https://service.elsevier.com for more information
Supplementary dataElsevier accepts electronic supplementary material (e-components) to support and enhance presentation of your scientific research. Supplementary files offer the Author additional possibilities to publish supporting applications, movies, animation sequences, high-resolution images, background datasets, sound clips and more. Supplementary files supplied will be published online alongside the electronic version of your article in Elsevier Web products, including ScienceDirect: https://www.sciencedirect.com. In order to ensure that your submitted material is directly usable, please ensure that data is provided in one of our recommended file formats. Authors should submit the material in electronic format together with the article and supply a concise and descriptive caption for each file. For more detailed instructions please visit our artwork instruction pages at https://www.elsevier.com/artworkinstructions.Footnotes should be used sparingly. Number them consecutively throughout the article. Many word processors can build footnotes into the text, and this feature may be used. Otherwise, please indicate the position of footnotes in the text and list the footnotes themselves separately at the end of the article. Do not include footnotes in the Reference list. Graphical abstract
The graphical abstract is mandatory at submission of the revised version of the paper. It will be included as the last figure in the PDF of the accepted manuscript. Please provide a figure legend for it and cite it in the Discussion section.
Technical Requirements
- Size: Please provide an image with a minimum of 531 x 1328 pixels (h x w) or proportionally more. The image should be readable at a size of 5 x 13 cm using a regular screen resolution of 96 dpi
- Font type and font size: Calibri 18
- Preferred file types: TIFF, EPS, PDF or MS Office files
- Content: the abstract should consist of one single panel
Content
KEEP IT SIMPLE
The graphical abstract should:
- Have a clear start and end, "reading" from top?to?bottom or left?to?right
- Emphasize the new findings from the current paper without including excess details from previous literature
- Avoid the inclusion of features that are more speculative (unless the speculative nature can be made apparent visually)
- Not be too text-heavy; most of the content should be in a graphical form
- Use simple labels
You can view Examples of Graphical Abstracts here
HighlightsHighlights are mandatory for this journal. They consist of a short collection of bullet points that convey the core findings of the article and should be submitted in a separate editable file in the online submission system. Please use 'Highlights' in the file name and include 3 to 5 bullet points (maximum 124 characters, including spaces, per bullet point). Footnotes
Footnotes should be used sparingly. Number them consecutively throughout the article. Many word processors can build footnotes into the text, and this feature may be used. Otherwise, please indicate the position of footnotes in the text and list the footnotes themselves separately at the end of the article. Do not include footnotes in the Reference list. Artwork Colour illustrations online
Please make sure that artwork files are in an acceptable format (TIFF, EPS or MS Office files) and with the correct resolution. Polaroid colour prints are not suitable. If, together with your accepted article, you submit usable colour figures then Elsevier will ensure, at no additional charge, that these figures will appear in colour on the Web (e.g. ScienceDirect and other sites. For further information on the preparation of electronic artwork, please see https://www.elsevier.com/artworkinstructions. Electronic artwork
General points
• Make sure you use uniform lettering and sizing of your original artwork.
• Embed the used fonts if the application provides that option.
• Aim to use the following fonts in your illustrations: Arial, Courier, Times New Roman, Symbol, or use fonts that look similar.
• Number the illustrations according to their sequence in the text.
• Use a logical naming convention for your artwork files.
• Provide captions to illustrations separately.
• Size the illustrations close to the desired dimensions of the published version.
• Submit each illustration as a separate file.
• Ensure that color images are accessible to all, including those with impaired color vision.
A detailed guide on electronic artwork is available.
You are urged to visit this site; some excerpts from the detailed information are given here.
Formats
If your electronic artwork is created in a Microsoft Office application (Word, PowerPoint, Excel) then please supply 'as is' in the native document format.
Regardless of the application used other than Microsoft Office, when your electronic artwork is finalized, please 'Save as' or convert the images to one of the following formats (note the resolution requirements for line drawings, halftones, and line/halftone combinations given below):
EPS (or PDF): Vector drawings, embed all used fonts.
TIFF (or JPEG): Color or grayscale photographs (halftones), keep to a minimum of 300 dpi.
TIFF (or JPEG): Bitmapped (pure black & white pixels) line drawings, keep to a minimum of 1000 dpi.
TIFF (or JPEG): Combinations bitmapped line/half-tone (color or grayscale), keep to a minimum of 500 dpi.
Please do not:
• Supply files that are optimized for screen use (e.g., GIF, BMP, PICT, WPG); these typically have a low number of pixels and limited set of colors;
• Supply files that are too low in resolution;
• Submit graphics that are disproportionately large for the content.
This journal encourages you to cite underlying or relevant datasets in your manuscript by citing them in your text and including a data reference in your Reference List. Data references should include the following elements: author name(s), dataset title, data repository, version (where available), year, and global persistent identifier. Add [dataset] immediately before the reference so we can properly identify it as a data reference. The [dataset] identifier will not appear in your published article. Preprint references
Where a preprint has subsequently become available as a peer-reviewed publication, the formal publication should be used as the reference. If there are preprints that are central to your work or that cover crucial developments in the topic, but are not yet formally published, these may be referenced. Preprints should be clearly marked as such, for example by including the word preprint, or the name of the preprint server, as part of the reference. The preprint DOI should also be provided. Reference style
Text: Indicate references by number(s) in square brackets in line with the text. The actual authors can be referred to, but the reference number(s) must always be given.
Example: '..... as demonstrated [3,6]. Barnaby and Jones [8] obtained a different result ....'
List: Number the references (numbers in square brackets) in the list in the order in which they appear in the text.
Examples:
Reference to a journal publication:
[1] J. van der Geer, J.A.J. Hanraads, R.A. Lupton, The art of writing a scientific article, J. Sci. Commun. 163 (2010) 51–59. https://doi.org/10.1016/j.Sc.2010.00372.
Reference to a journal publication with an article number:
[2] J. van der Geer, J.A.J. Hanraads, R.A. Lupton, 2018. The art of writing a scientific article. Heliyon. 19, e00205. https://doi.org/10.1016/j.heliyon.2018.e00205.
Reference to a book:
[3] W. Strunk Jr., E.B. White, The Elements of Style, fourth ed., Longman, New York, 2000.
Reference to a chapter in an edited book:
[4] G.R. Mettam, L.B. Adams, How to prepare an electronic version of your article, in: B.S. Jones, R.Z. Smith (Eds.), Introduction to the Electronic Age, E-Publishing Inc., New York, 2009, pp. 281–304.
Reference to a website:
[5] Cancer Research UK, Cancer statistics reports for the UK. http://www.cancerresearchuk.org/aboutcancer/statistics/cancerstatsreport/, 2003 (accessed 13 March 2003).
Reference to a dataset:
[dataset] [6] M. Oguro, S. Imahiro, S. Saito, T. Nakashizuka, Mortality data for Japanese oak wilt disease and surrounding forest compositions, Mendeley Data, v1, 2015. https://doi.org/10.17632/xwj98nb39r.1.
Reference to software:
[7] E. Coon, M. Berndt, A. Jan, D. Svyatsky, A. Atchley, E. Kikinzon, D. Harp, G. Manzini, E. Shelef, K. Lipnikov, R. Garimella, C. Xu, D. Moulton, S. Karra, S. Painter, E. Jafarov, S. Molins, Advanced Terrestrial Simulator (ATS) v0.88 (Version 0.88), Zenodo, March 25, 2020. https://doi.org/10.5281/zenodo.3727209.
[dataset] [6] M. Oguro, S. Imahiro, S. Saito, T. Nakashizuka, Mortality data for Japanese oak wilt disease and surrounding forest compositions, Mendeley Data, v1, 2015. http://dx.doi.org/10.17632/xwj98nb39r.1. Data Processing Policy
Authors should reduce postacquisition processing of data. If deemed necessary for proper evaluation of the manuscript, authors will be required to make the original unprocessed data available to the editors of the journal. Data visualization
Include interactive data visualizations in your publication and let your readers interact and engage more closely with your research. Follow the instructions here to find out about available data visualization options and how to include them with your article. Research data
This journal encourages and enables you to share data that supports your research publication where appropriate, and enables you to interlink the data with your published articles. Research data refers to the results of observations or experimentation that validate research findings, which may also include software, code, models, algorithms, protocols, methods and other useful materials related to the project.
Below are a number of ways in which you can associate data with your article or make a statement about the availability of your data when submitting your manuscript. If you are sharing data in one of these ways, you are encouraged to cite the data in your manuscript and reference list. Please refer to the "References" section for more information about data citation. For more information on depositing, sharing and using research data and other relevant research materials, visit the research data page.
Data linkingIf you have made your research data available in a data repository, you can link your article directly to the dataset. Elsevier collaborates with a number of repositories to link articles on ScienceDirect with relevant repositories, giving readers access to underlying data that gives them a better understanding of the research described.
There are different ways to link your datasets to your article. When available, you can directly link your dataset to your article by providing the relevant information in the submission system. For more information, visit the database linking page.
For supported data repositories a repository banner will automatically appear next to your published article on ScienceDirect.
In addition, you can link to relevant data or entities through identifiers within the text of your manuscript, using the following format: Database: xxxx (e.g., TAIR: AT1G01020; CCDC: 734053; PDB: 1XFN).
Submission ChecklistThe following list will be useful during the final checking of an article prior to sending it to the journal for review. Please consult this Guide for Authors for further details of any item.
Ensure that the following items are present:
- One author has been designated as the corresponding author with contact details:
- E-mail address
- Full postal address
- All necessary files have been uploaded, and contain:
- Keywords
- All figure captions in the suitable style
- All tables (including title, description, footnotes in the suitable style)
- Further considerations:
- Manuscript has been 'spell-checked' and 'grammar-checked'
- Formatting guidelines have been applied for revised submissions (Please see Atherosclerosis Style Checklist)
- All references mentioned in the Reference list are cited in the text, and vice versa
- Permission has been obtained for use of copyrighted material from other sources (including the Internet)
- Printed version of figures (if applicable) in color or black-and-white:
- Indicate clearly whether or not color or black-and-white in print is required.
For any further information please visit our customer support site at https://service.elsevier.com.
Co-submissionThis journal enables you to co-submit a data, methods or protocol article alongside your original research article. The Co-submission article describes either research data, methods or protocols related to your original research article and will be considered for publication in either Data in Brief or in MethodsX after peer review. When submitting your original research article, you may attach your Co-submission article by selecting the Item type: Data in Brief or MethodsX. In case both your original research article and your Co-submission article get accepted for publication, they will be linked together on ScienceDirect.
Please note that Co-submission articles will only be considered for review if they follow the below submission templates:
- Data article template (Data in Brief)
- Methods article template (MethodsX)
- Protocol article template (MethodsX)
To foster transparency, we encourage you to state the availability of your data in your submission. This may be a requirement of your funding body or institution. If your data is unavailable to access or unsuitable to post, you will have the opportunity to indicate why during the submission process, for example by stating that the research data is confidential. The statement will appear with your published article on ScienceDirect. For more information, visit the Data Statement page.
Atherosclerosis policy on the use of proper terminology when referring to intima-media thickness (IMT)
Atherosclerosis has recently embraced a new editorial policy to clarify the use of proper terminology when referring to intima-media thickness (IMT): IMT should be referred to as "arterial injury" or "arteriopathy", not atherosclerosis. For more details, please see the following letter to the editor and reply published in Atherosclerosis
"IMT is not atherosclerosis", Spence 2020 (https://doi.org/10.1016/j.atherosclerosis.2020.09.016) .
"Carotid intima-media thickness should not be referred to as subclinical atherosclerosis: A recommended update to the editorial policy at Atherosclerosis", Raggi and Stein 2020 (https://doi.org/10.1016/j.atherosclerosis.2020.09.015) .
Additional information Changes to authorshipAuthors are expected to consider carefully the list and order of authors before submitting their manuscript and provide the definitive list of authors at the time of the original submission. Any addition, deletion or rearrangement of author names in the authorship list should be made only before the manuscript has been accepted and only if approved by the journal Editor. To request such a change, the Editor must receive the following from the corresponding author: (a) the reason for the change in author list and (b) written confirmation (e-mail, letter) from all authors that they agree with the addition, removal or rearrangement. In the case of addition or removal of authors, this includes confirmation from the author being added or removed.
Only in exceptional circumstances will the Editor consider the addition, deletion or rearrangement of authors after the manuscript has been accepted. While the Editor considers the request, publication of the manuscript will be suspended. If the manuscript has already been published in an online issue, any requests approved by the Editor will result in a corrigendum. Copyright
Upon acceptance of an article, authors will be asked to complete a 'Journal Publishing Agreement' (see more information on this). An e-mail will be sent to the corresponding author confirming receipt of the manuscript together with a 'Journal Publishing Agreement' form or a link to the online version of this agreement.
Subscribers may reproduce tables of contents or prepare lists of articles including abstracts for internal circulation within their institutions. Permission of the Publisher is required for resale or distribution outside the institution and for all other derivative works, including compilations and translations. If excerpts from other copyrighted works are included, the author(s) must obtain written permission from the copyright owners and credit the source(s) in the article. Elsevier has preprinted forms for use by authors in these cases.
For gold open access articles: Upon acceptance of an article, authors will be asked to complete a 'License Agreement' (more information). Permitted third party reuse of gold open access articles is determined by the author's choice of user license.
Author rights
As an author you (or your employer or institution) have certain rights to reuse your work. More information.
You are requested to identify who provided financial support for the conduct of the research and/or preparation of the article and to briefly describe the role of the sponsor(s), if any, in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. If the funding source(s) had no such involvement, it is recommended to state this. Article Transfer Service
This journal is part of our Article Transfer Service. This means that if the Editor feels your article is more suitable in one of our other participating journals, then you may be asked to consider transferring the article to one of those. If you agree, your article will be transferred automatically on your behalf with no need to reformat. Please note that your article will be reviewed again by the new journal. More information. Green Open Access
Authors can share their research in a variety of different ways and Elsevier has a number of green open access options available. We recommend authors see our green open access page for further information. Authors can also self-archive their manuscripts immediately and enable public access from their institution's repository after an embargo period. This is the version that has been accepted for publication and which typically includes author-incorporated changes suggested during submission, peer review and in editor-author communications. Embargo period: For subscription articles, an appropriate amount of time is needed for journals to deliver value to subscribing customers before an article becomes freely available to the public. This is the embargo period and it begins from the date the article is formally published online in its final and fully citable form. Find out more.
After acceptance
Use of the Digital Object IdentifierThe Digital Object Identifier (DOI) may be used to cite and link to electronic documents. The DOI consists of a unique alpha-numeric character string which is assigned to a document by the publisher upon the initial electronic publication. The assigned DOI never changes. Therefore, it is an ideal medium for citing a document, particularly 'Articles in press' because they have not yet received their full bibliographic information. Example of a correctly given DOI (in URL format; here an article in the journal Physics Letters B): http://dx.doi.org/10.1016/j.physletb.2010.09.059
When you use a DOI to create links to documents on the web, the DOIs are guaranteed never to change.
Elsevier will do everything possible to get your article corrected and published as quickly and accurately as possible. Therefore, it is important to ensure that all of your corrections are sent back to us in one communication. Subsequent corrections will not be possible, so please ensure your first sending is complete
ReprintsPDF offprints are provided free of charge. No reprints are provided free of charge. Reprints (50 copies minimum) can be ordered at quoted prices on order forms sent out together with the proofs. Online proof correction
To ensure a fast publication process of the article, we kindly ask authors to provide us with their proof corrections within two days. Corresponding authors will receive an e-mail with a link to our online proofing system, allowing annotation and correction of proofs online. The environment is similar to MS Word: in addition to editing text, you can also comment on figures/tables and answer questions from the Copy Editor. Web-based proofing provides a faster and less error-prone process by allowing you to directly type your corrections, eliminating the potential introduction of errors.
If preferred, you can still choose to annotate and upload your edits on the PDF version. All instructions for proofing will be given in the e-mail we send to authors, including alternative methods to the online version and PDF.
We will do everything possible to get your article published quickly and accurately. Please use this proof only for checking the typesetting, editing, completeness and correctness of the text, tables and figures. Significant changes to the article as accepted for publication will only be considered at this stage with permission from the Editor. It is important to ensure that all corrections are sent back to us in one communication. Please check carefully before replying, as inclusion of any subsequent corrections cannot be guaranteed. Proofreading is solely your responsibility.
Author inquiries
Visit the Elsevier Support Center to find the answers you need. Here you will find everything from Frequently Asked Questions to ways to get in touch.
You can also check the status of your submitted article or find out when your accepted article will be published. SPECIAL GUIDELINES Clinical Trials
The International Committee of Medical Journal Editors (ICMJE) defines a clinical trial as any research project prospectively assigning human participants to intervention or comparison groups to study the cause-and-effect relationship between an intervention and a health outcome. Interventions include but are not limited to drugs, surgical procedures, devices, behavioral treatments, process-of-care changes, and the like. All manuscripts reporting clinical trials, must include a copy of the trial protocol including the complete statistical analysis plan, a flow diagram (CONSORT flow diagram), and a completed trial checklist (CONSORT checklist). The trial registration number must be included on the title page of the manuscript reporting a registered clinical trial and in the Materials and methods section. Failure to do so will prevent entry to the peer review process.
Registration of clinical trial
Registration in a public trials registry is a condition for publication of clinical trials in Atherosclerosis in accordance with International Committee of Medical Journal Editors (ICMJE) recommendations. Trials must be registered at or before the onset of patient enrolment. Purely observational studies (those in which the assignment of the medical intervention is not at the discretion of the investigator) will not require registration.
Clinical trial results
In line with the position of the ICMJE, Atherosclerosis will not consider results posted in the same clinical trials registry in which primary registration resides to be prior publication if the results posted are presented in the form of a brief structured (less than 500 words) abstract or table. However, divulging results in other circumstances (e.g., investors' meetings) is discouraged and may jeopardise consideration of the manuscript. Authors should fully disclose all posting in registries of results of the same or closely related work.
Randomized control trials
Reports of randomised trials must conform to CONSORT 2010 guidelines. All manuscripts reporting randomized clinical trials, must include a copy of the trial protocol including the complete statistical analysis plan, a flow diagram (CONSORT flow diagram), and a completed trial checklist (CONSORT checklist).
Guidelines for genetic association papersAtherosclerosis is interested in publishing genetic association papers that present data that is novel, statistically robust, clinically relevant and that add significantly to the field. Authors are advised to follow the reporting guidelines outlined in the STREGA Statement (http://www.strega-statement.org) [1], and to achieve this, the following criteria should be met.
1. All the following aspects should be addressed appropriately and Methods used should be reported:
a) Population stratification should be addressed in case of admixed populations;
b) Test on Hardy-Weinberg-Equilibrium must be carried out and the p value reported;
c) LD-structure between SNPs (if multiple SNPs are reported) must be presented;
d) Genotyping errors / call rate must be reported;
e) Appropriate correction for multiple testing (if multiple independent SNPs are reported) must be included;
f) Possible relatedness between studied subjects must be documented and addressed if present.
2. All papers must include a power calculation to estimate the effect the size the study has the power to detect, based on sample size and minor allele frequency of the included SNPs. If power calculations are not included the paper is likely to be rejected without review. It should be stated whether or not power calculations were performed before or after study completion. Comment: The study should have an adequate sample size. Ideally, power calculations should have been performed before conducting the study since post-hoc power calculations are often a self-fulfilling prophecy. It should be stated whether or not power calculations were performed before or after study completion. Several programs are available to perform power and/or sample size calculations for genetic association studies, e.g. the "Genetic Power Calculator" (http://pngu.mgh.harvard.edu/~purcell/gpc) [2], and see table 1 below. Sample size and /or Power calculations on two-stage designs can be calculated e.g. by using the program CATS (http://www.sph.umich.edu/csg/abecasis/CaTS) [3] for case-control studies and QpowR (https://www.msu.edu/~steibelj/JP_files/QpowR.html) for studies on quantitative traits. Since genetic association studies often involve more complex study designs involving meta-analysis or several replication stages, simple answers on required sample sizes cannot be given. Authors are advised, however, to keep this issue in mind and give a good rationale, if the study is clearly underpowered.
3. For any novel association a replication study must be included in the submitted manuscript. Any novel association not including a replication study may be rejected without review. Comment: The presentation of novel association results requires replication in most cases, if appropriate replication studies exist. However, if the first study has already an appropriate sample size (considering that very large studies with several thousands of individuals are available) and if the results show a strong association, it might not be necessary to provide a replication. Furthermore, giving additional evidence from other sources could replace replication studies, if they are convincing, e.g. results from functional experiments. Meta-analysis on the discovery stage or other outstanding studies do also not require replication in every case, but it should be clear that these are exceptional cases and have to discussed in that way to be acceptable for publication.
4. For any association study replicating a previously published finding, there should be sufficient novelty to add significantly to the literature. This could include confirming the effect size in a different ethnic group, or extending the association observations to additional intermediate traits or disease groups. Any study not having sufficient novelty is likely to be rejected without review.
5. We require all SNPs to have their designated RS number and for the numbering of base pair changes and amino acid changes and gene symbols to be using agreed nomenclature. For example see the following website: http://www.hgvs.org/mutnomen.
6. Generally, authors should present the rationale as to why gene regions and SNPs have been selected. Association studies using SNPs where previous studies have demonstrated that the base change has an effect on protein function or gene expression will be favored over those using SNPs where no functionality has been previously determined. Studies using a tagSNP approach will also be considered, where these add additional data to the already known variations, in order to further explain observed associations.
References
[1] Little J et al: Strengthening the Reporting of Genetic Association Studies (STREGA): an extension of the STROBE statement. PLoS Med. 2009 Feb 3;6(2):e22.
[2] Purcell S, et al. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics 2003, 19(1):149-150.
[3] Skol AD et al. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet (2006) 38:209-13.
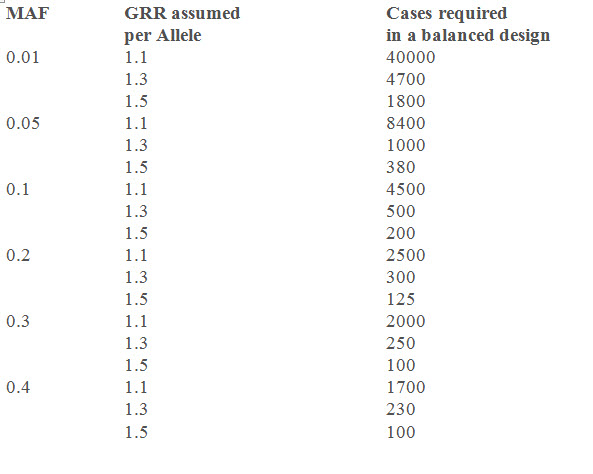
In the following table, some sample sizes are given, calculated from the "Genetic Power Calculator", assuming an alpha-level of = 0.05, an additive inheritance model, an assumed prevalence of disease of 30% and a power of 80% for a balanced case-control study (1:1 case:control ratio) for varying minor allele frequencies (MAF) and genetic relative risks (GRR). Relative risks of between 1.1 and 1.3 are in the range that can be expected in genetic association studies on complex diseases.
Guidelines for meta-analysesIn principle, literature-based meta-analyses should be reported in that way, that any interested researcher is able to reproduce the results. To ensure this, authors are strongly advised to follow the guidelines listed below and are further encouraged to use the PRISMA (http://www.prisma-statement.org/PRISMAStatement/Default.aspx) and the MOOSE statements (http://jama.ama-assn.org/cgi/content/full/283/15/2008) as a guide. Therefore, as much information as needed should be provided. However, for the average reader only the most mandatory information should be reported in the main paper with additional information given in the Supplementary Material.
1. Specification of objective and primary study outcome. If there are previous meta-analyses on the same outcome available, the authors should specify clearly the differences and added value of their meta-analysis in a separate section ("Added value to previous meta-analysis on the same topic").
2. Detailed specification of search strategy, study selection strategy (including approaches to reach unpublished studies) and eligibility criteria for studies. It is highly recommended to use a graphical Flow Chart (templates available at http://www.prisma-statement.org/PRISMAStatement/Default.aspx).
3. Description of possible sources of bias and confounding and strategies to prevent them. This includes:
- Bias in individual studies
- Bias across studies (e.g. publication bias, selective reporting within studies)
- Quality and comparability of studies (study types, study outcomes, sample size)
4. Description of Statistical Methods:
- What is the primary summary measure (Difference in Mean, OR, etc.)? How was it extracted from the individual studies (e.g. calculated from raw numbers or tables or taken as reported)
- Methods to assess heterogeneity and bias
- Methods used for the combined analysis (fixed effects, random effects) including a rationale for using this method.
5. Reporting of results:
- Individual study characteristics (including sample size, study type, population/ethnicity, primary outcome, reference)
- Individual study results (effect estimates including confidence intervals or standard errors). Graphical presentations is preferred (Forest plots).
- Meta-analysis results: Combined effect estimate, confidence intervals, some measure of heterogeneity, results of bias assessment (preferably using graphical presentations, e.g. Funnel plot)
6. Additional for meta-analysis of genetic association studies: meta-analysis on a single SNP with certain selected outcomes suffer from the problem that they completely ignore the other genetic variability within a certain gene region. Many of these meta-analyses also completely ignore already available results from genome-wide association (GWA) studies on the investigated outcomes. These GWA studies might not have studied the very SNP of interest but highly correlated ones in the same genetic region which can add valuable information to the meta-analysis. The authors must either discuss the findings from these GWAS or - even much better - approach the authors from these GWAS for a lookup of the meta-analyzed SNPs. Meta analyses that do not cover these issues will be rejected without review. Furthermore, these studies have to report the following information:
- Specification of the genes / polymorphisms (rs numbers) and rationale for selection of the specific polymorphisms
- Genotyping methods in each individual study
- Genotype characteristics (genotyping success rate, minor allele frequency, frequencies of genotypes, Hardy-Weinberg-equilibrium).